Abstract
Post-transplant lymphoproliferative disorder (PTLD) represents a spectrum of lymphoproliferative disorders and is a serious complication of pediatric transplantation. The majority of PTLD are associated with Epstein Barr virus (EBV) and the characteristic EBV+ B cell lymphomas are the leading post-transplant malignancy in children. EBV+ PTLD remains a formidable issue in pediatric transplantation and is thought to result from impaired immunity to EBV as a result of immunosuppression. However, the key viral and immune factors that determine whether EBV+ PTLD develops remain unknown. Recently, there has been much interest in developing biomarkers in order to improve and achieve more personalized approaches, in the clinical diagnosis, management, and treatment of EBV+ PTLD. Here, we review the status of immune-, viral-, and B cell lymphoma-derived candidates for biomarkers of EBV+ PTLD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
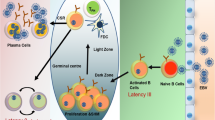
Kidney transplantation is the treatment of choice for children with a variety of end-stage renal diseases [1]. However, life-long immunosuppression is required to prevent immune-mediated allograft rejection, and this is associated with several serious complications in children, including increased risk of infection and de novo malignancies. In pediatric transplant recipients, the most common post-transplant malignancy is associated with the Epstein Barr virus (EBV). EBV is a widely disseminated gamma herpes virus that, in immunocompetent individuals, is linked with both lymphoid and epithelial malignancies including Burkitt’s lymphoma, Hodgkin’s disease, gastric adenocarcinomas, nasopharyngeal carcinomas, and lymphomas in immunocompromised or immunosuppressed individuals. The latter are classified within a heterogeneous collection of abnormal lymphoproliferations termed post-transplant lymphoproliferative disorder (PTLD), the majority of which are of B cell origin and are associated with EBV [2,3,4]. EBV+ B cell lymphomas in PTLD can be rapidly progressing and potentially fatal, with mortality approaching 50%. In fact, EBV+ PTLD is the most common malignancy in the pediatric transplant population with the incidence ranging from ~ 3–7% in pediatric renal transplant recipients depending on the series [5,6,7]. Pediatric transplant recipients, in particular, are especially susceptible to EBV+ PTLD since they are often immunologically naïve prior to transplantation and may acquire the virus from the donor organ in the setting of maximal immunosuppression. Moreover, a notable proportion of pediatric renal recipients will develop EBV DNAemia, the clinical significance of which is enigmatic. Finally, current treatment options for EBV+ PTLD include reduction or elimination of immunosuppression, chemotherapy, radiotherapy, the anti-CD20 antibody Rituximab, and surgical resection when feasible [8]. However, there is no consensus on the optimal treatment because the efficacy of these approaches is highly variable, with relapse rates and treatment-related mortality a major concern, and because we cannot predict which treatment strategy will best benefit individual patients [9,10,11,12]. Given these challenges, there is an urgent, unmet need for the development of minimally invasive biomarkers to improve personalized diagnosis, management, and treatment of EBV+ PTLD in pediatric transplant recipients. At present, no such biomarkers have been established and validated. Here, we discuss the current state of an ongoing investigation to elucidate cellular, molecular, and genetic characteristics associated with EBV infection and EBV+ PTLD, and the possibility of their application as biomarkers (Fig. 1).
Viral, immune, and lymphoma-derived biomarker candidates for EBV+ PTLD. Potential biomarkers for EBV+ PTLD include miRNA and either cell-associated or extracellular DNA from the virus; molecules, including cytokines, miRNA, soluble light chain, and sCD30 produced by the B lymphoma cells, genomic variants, or polymorphisms from the host cell or EBV DNA, and alterations in immune cell frequency or function
EBV life cycle and the immune response
EBV is typically transmitted through the saliva from an infected individual to the host. In transplantation, another important mode of transmission is via the graft, particularly in children who may be EBV seronegative but receive an organ from an EBV-seropositive adult. Following infection, EBV enters a lytic phase that is accompanied by viral replication in the oropharynx involving epithelial cells and B cells that culminates in the production of new viral progeny. Viral particles are shed and can go on to infect other cells or they can be transmitted to another host. In immunocompetent individuals, the lytic phase is asymptomatic, though in adolescents and young adults it can result in infectious mononucleosis (IM), which is usually self-limiting. Eventually, EBV transitions to a chronic, latent phase in which the virus is harbored as an episome in a subset of memory B cells for the lifetime of the host. Periodically, reactivation of the virus can occur possibly triggered by antigen engagement of the B cell receptor of the infected cell; however, the exact mechanisms surrounding this process remain unclear.
There is extensive evidence that the T cell response to EBV is critical for protection against the virus [13]. Each of the phases of the viral life cycle is associated with expression of specific viral proteins that are capable of eliciting a potent cellular immune response dominated by T cells, primarily CD8+ T cells, although CD4+ T cells also play a role. Extensive analysis of T cell responses to EBV in healthy donors and IM patients, using in vitro assays such as ELISPOT, cytotoxicity assays, or cytokine production, and enumeration with MHC/peptide tetramers, has catalogued a large number of HLA-restricted immunodominant epitopes derived from both latent and lytic cycle viral proteins [14]. However, the associated responses in transplant recipients, and especially those in patients that go on to develop EBV+ PTLD, are poorly understood. Nevertheless, the impairment of T cell function by immunosuppression is thought to be a major factor contributing to the development of EBV+ PTLD.
Current approaches for monitoring EBV in transplant recipients
EBV serology is routinely utilized to assess the status of viral infection, and antibodies against latent and lytic cycle proteins can be produced [15]. However, the immunosuppression administered to transplant recipients complicates the interpretation of post-transplant serologies owing to alterations or inhibition of the immune response. Consequently, PCR-based methods to directly quantitate EBV DNA are used extensively to monitor the viral load during the post-transplant period, particularly in high-risk donor-recipient combinations which occurs when EBV-naïve children receive a graft from an EBV-seropositive donor. While EBV+ PTLD can arise at any time post-transplantation, the incidence is highest in the first 1–2 years post-transplant, so monitoring tends to be most frequent during this period. In addition, the onset of clinical features symptomatic of EBV+ PTLD can prompt EBV PCR testing, and it can also be utilized to monitor response to treatment for EBV+ PTLD. Currently, there is no standardized assay for EBV quantitation and multiple PCR-based platforms have been used, including those developed in-house or at the center’s designated clinical lab. Thresholds for the determination of elevated EBV viral load are established by individual labs, but this also hinders the ability to compare the results across centers. The development of a WHO international standard for EBV should contribute to improved harmonization of PCR-based quantitation of EBV across centers [16]. However, it remains unclear what the most informative compartment is to analyze for the quantitation of virus. Most studies utilize either whole blood or plasma, but others have utilized serum, peripheral blood mononuclear cells (PBMC), or isolated B cells for the assessment of EBV viral load. Thus, a multitude of studies have compared the measurement of EBV viral load in samples from various compartments [17,18,19,20,21] as well as in different organs [19, 21,22,23,24]. Across these studies, the viral load cutoff can vary markedly with respect to numerical range, the denominator used (per μg of DNA, per 105 or 106 cells, per ml), and the different types of organ transplants. It will also be important to evaluate this information in the context of whether the purpose is surveillance or assessment of viral load when PTLD is suspected.
The clinical significance of observed elevations in EBV viral load has been the subject of intense interest in the field, yet there remains no consensus on how to apply this information to diagnosis and management of EBV+ PTLD. DNAemia does not necessarily portend the onset of EBV+ PTLD. Whereas prior studies show the majority of patients with EBV+ PTLD had elevations in EBV viral load prior to clinical diagnosis [25], and higher first positive or peak levels of virus [26, 27], not all patients with DNAemia go on to develop EBV+ PTLD. In fact, some patients manifest prolonged viral elevations and have been termed chronic high viral load (CHL) carriers, but do not progress to EBV+ PTLD [28, 29]. Green et al. showed that CHL patients were more likely to progress to EBV+ PTLD if they were cardiac recipients as compared with liver recipients, illustrating the complexity of this problem [30]. A recent study of pediatric kidney transplant recipients indicates that 8% became CHL but none went on to develop late-onset EBV+ PTLD [31]. These results suggest that the proportion of pediatric kidney recipients with CHL that ultimately develop PTLD is less than seen in other solid organ transplants. Thus, the interpretation and clinical management decisions, in the face of elevated EBV load, are challenging, and additional monitoring approaches are needed to assess the risk, or impending onset, of EBV+ PTLD.
Biomarkers
Biomarkers have been defined by the National Institutes of Health Biomarkers Definitions Group [32] as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.” Biomarker assessment has become an increasingly common feature of clinical studies and may inform on the risk of disease, diagnosis, clinical outcome, and response to treatment depending on study design [33]. Less invasive sampling, as with blood, is preferable but analysis of tumor specimens in EBV+ PTLD has also been investigated. Currently, there are no established biomarkers for use in EBV+ PTLD. Discovery, validation, and reproducibility testing of biomarkers are a rigorous, multi-step process, and a variety of statistical tests must be applied to establish a biomarker as reviewed extensively by others [33, 34]. Here, we focus on recent studies of biomarker candidate molecules for determining risk, diagnosis, and response to the treatment of EBV+ PTLD. We have categorized EBV+ PTLD biomarkers candidates according to their origin, whether it be the virus itself, host genomics, the immune response to EBV, or the virally infected B lymphoma cells.
Viral nucleic acids and proteins
Detection of EBV nucleic acids and proteins in tissues for diagnosis
Viral nucleic acids or proteins are useful in establishing the presence of EBV in tissues for diagnostic purposes. Along these lines, in situ hybridization of Epstein Barr encoding region (EBER) is the standard method for the detection of EBV in tissue sections. The presence of EBER is consistent with the latent EBV infection, as is seen in EBV+ PTLD, and can help localize the virus to specific cell types within the lesion. Immunohistochemical staining for latent membrane protein 1 (LMP1), an EBV-encoded latent cycle protein, is also used to identify EBV-infected cells in tissue specimens, though this methodology may be less conclusive given the sensitivity of the anti-LMP1 antibodies that are an available and potentially variable expression of LMP1 in tumors.
Detection of EBV nucleic acids and proteins in blood as predictors of PTLD or at the time of diagnosis
miRNA are small, non-coding RNA that are important in post-transcriptional regulation of cellular genes and have attracted interest as biomarkers, in part, due to their stability in blood. Of note, EBV was the first virus shown to encode miRNA [35]. However, despite the growing literature on the importance of EBV miRNA in the process of B cell transformation and proliferation, there are relatively few studies that have addressed their potential as biomarkers for EBV+ PTLD. Hassan et al. [36] examined 42 EBV-encoded miRNA originating from the BART and BHRF viral open reading frames in pediatric renal transplant recipients. They noted several BART-derived miRNA, including BART7-3p, 15, 9-3p, 1-3p, and 3-3p, were only detected in CHL patients (n = 10 analyzed) and IM patients, compared with transplant recipients that had previously resolved an EBV infection. One CHL patient went on to develop EBV+ PTLD many years later and had markedly increased BART2-5p, compared with CHL patients, prior to disease onset. A few studies have examined the EBV miRNome within the PTLD tumor tissue itself. Navari et al. [37] found miRNA expression in tumor tissue was primarily of cellular origin, but the EBV miRBHRF-1-2 was detected in diffuse large B cell lymphoma type PTLD as compared with EBV+ Burkitt’s lymphoma tissues. miRNA profiles were examined in CNS-associated EBV+ PTLD as compared with systemic EBV+ PTLD to gain insight into viral and cellular features that may distinguish them [38]. BART-encoded and BHRF1-encoded miRNA were identified in most of the EBV+ PTLD specimens but 28 host cell–derived miRNA were found to be differentially expressed between systemic and CNS type PTLD. Interestingly, the CNS PTLD specimens could be divided into two subtypes, one that was more similar to the systemic PTLD and another that was unique to the CNS. Whereas analysis of latent cycle genes failed to distinguish the CNS and systemic PTLD specimens, at least one lytic gene could be identified in all EBER+ lymphomas, despite the presence of the type III latency pattern that is a characteristic of EBV+ PTLD.
Expression of the lytic cycle gene BZLF1 and the latent cycle gene LMP2A was analyzed in the blood of PTLD patients, transplant recipients without PTLD, and non-transplanted subjects with EBV DNAemia [39]. The ratio between LMP2A and BZLF1 was not different between PTLD and non-PTLD transplant recipients but was lower than in non-transplanted subjects. Habib et al. [40] measured the ZEBRA protein, a product of the BZLF1 gene of EBV, in the serum of 35 patients with PTLD. Soluble ZEBRA was detected in 60% of PTLD cases and was present in significantly higher levels in early-onset PTLD patients, but not late-onset PTLD, compared with subjects who did not develop PTLD.
Expression of the latent cycle genes EBNA3c, LMP1, and LMP2A/B, and the lytic cycle–associated gene gp350 was variable in the blood of 11 cardiac recipients with PTLD with a mixture of lytic and latent patterns [41]. The most common signature showed evidence of latency III and lytic cycle activity, similar to what was observed in healthy subjects with elevated viral levels but also observed in transplant recipients without PTLD. Collectively, these studies indicate that gene expression of viral genes alone does not correlate with the presence of EBV+ PTLD. Additional studies are needed to determine whether viral gene expression can be used to predict the onset of EBV+ PTLD. Along these lines, a current ongoing multi-center, prospective clinical study in pediatric transplant recipients is evaluating whether the presence of two gain of function mutations in LMP1 [42] is associated with an increased risk of EBV+ PTLD (https://clinicaltrials.gov/ct2/show/NCT02182986). More broadly, next-generation sequencing approaches may facilitate the identification of viral genomic variations and/or mutations that are associated with the development of EBV+ PTLD and could potentially lead to new candidate biomarkers.
Other viruses that may be informative in the diagnosis of PTLD
The Quake laboratory analyzed the human virome in cell-free circulating DNA in the plasma of 96 heart and lung transplant recipients [43], and tracked the impact of immunosuppression and antiviral prophylaxis on viral levels. Interestingly, specific viral families were associated with different levels of drug therapy. When immunosuppression and antiviral medication were low, herpesvirales and caudovirales were the predominant viral families, whereas at high drug levels, anelloviridae was dominant. Patients with biopsy-proven rejection had significantly lower levels of anelloviruses than patients with no rejection. Together, these findings suggest that anelloviruses load may be a surrogate marker of immunocompetence with high levels of anelloviruses indicative of overimmunosuppression. Interestingly, metagenomic shotgun sequencing of formalin-fixed paraffin-embedded tissues from PTLD patients [44] showed that more than 50% of the specimens contained anellovirus sequences, and the anellovirus levels, but not EBV levels, were associated with death within 5 years in a univariate analysis. These findings indicate that changes in the virome, and even specific viruses such as anelloviruses, may provide indirect measures of immunosuppression and immune status which can promote the development of EBV+ PTLD.
Host
B cell–derived miRNA
While EBV encodes its own miRNA, it can also modulate the miRNA profile of the host cell it has infected. Harris-Arnold et al. [45] demonstrated that the establishment of a latent infection by EBV in human B cells markedly alters the expression of > 100 host cell miRNA, including miR155, which has been shown to promote the development of B cell malignancies [46]. Another miRNA, miR-194, was determined to participate in the regulation of IL-10 [45], a known autocrine growth factor for EBV+ B cell lymphomas [47]. More recently, it has been demonstrated that EBV-infected cells can release exosomes, cell-derived vesicles, containing miRNA, into the extracellular milieu suggesting that they may be a source of miRNA in the blood. Thus, like viral miRNA, EBV-infected B cell–derived miRNA are a family of molecules that warrant further exploration as biomarkers for EBV+ PTLD, particularly since miRNA have also shown promise as biomarkers in other EBV malignancies, including nasopharyngeal carcinoma [48, 49].
Genetic polymorphisms and HLA associations with EBV+ PTLD
Cytokines are important in mediating and regulating immune responses to viruses and tumor cells. Several single nucleotide polymorphisms have been defined within regulatory regions of cytokine genes that are linked to either high or low levels of production of the cytokine. Early studies examined cytokine gene polymorphisms in transplant recipients to determine whether either high- or low-production genotypes for specific cytokines were associated with the development of EBV+ PTLD. While some provocative associations were observed, some non-confirmatory or conflicting findings were also reported leaving the utility of cytokine gene polymorphism as potential biomarkers for the risk of EBV+ PTLD unresolved. For example, IFN-γ production by T cells and natural killer (NK) cells is known to be important in antiviral responses and a genotype linked to low IFN-γ production was associated with early onset of EBV+ PTLD [50]. However, this association was not confirmed in other studies [51, 52], though associations between low-production genotypes of IL-10 and TGF-β were noted in PTLD patients [52]. Moreover, McAuley [53] reported that a specific variant allele within the promoter of TNF-α was more frequent in EBV+ PTLD patients, but no differences were seen in polymorphic regions of lymphotoxin, IL-1-α, IL-6, IL-10, TNF receptor (R) I or II, and IL-1R and IL-10R. Therefore, at present, there is no conclusive cytokine gene polymorphism or group of polymorphisms that are indicative of the increased risk of development of EBV+ PTLD.
The possible link between HLA type and susceptibility to EBV+ PTLD has also been examined. The HLA of transplant recipients could potentially impact the quality and magnitude of the immune response to EBV, due to variable efficacy in the presentation of EBV-derived peptides and recognition by antiviral effector cells. Several HLA of either donor or recipient have been linked to increased, or decreased, susceptibility to EBV+ PTLD and have been reviewed elsewhere [54,55,56]. These findings underscore the challenge of biomarker analyses in EBV+ PTLD owing to the complexity of the disease, the variety of organs transplanted, and the difficulty in achieving large cohort sizes that yield conclusive, broadly applicable findings.
Immune response
Identification of EBV-specific T cells and characterization of T cell responses to EBV in PTLD patients
A protective immune response to a primary EBV infection in healthy individuals is associated with the expansion of significant populations of CD4+ and CD8+ T cells that are specific for well-defined immunodominant epitopes of both lytic and latent viral genes. Eventually, the EBV-specific T cell population contracts and memory T cells are generated that provide ongoing protection to the latent, persistent virus.
Because impaired, or altered, T cell responses to EBV are thought to be an important component of the pathogenesis of EBV+ PTLD, monitoring EBV-specific T cells in transplant recipients could reveal new biomarkers for the risk of EBV+ PTLD. One possibility that has been considered is that the generation or maintenance of EBV-specific T cells is diminished in those individuals that go onto to develop EBV+ PTLD. MHC/peptide multimers are complexes of HLA molecules carrying a specific peptide derived from a known immunodominant EBV epitope. These fluorescently tagged multimers, in combination with flow cytometry, can be used to identify antigen-specific T cells and, therefore, assess the frequency of T cells for known lytic and latent antigens. Early studies with MHC class I/peptide tetramers established that pediatric liver and kidney transplant recipients without PTLD had similar frequencies of CD8+ T cells specific for peptides derived from BZLF1, EBNA3A, and BMLF1 as those reported in non-transplanted, healthy seropositive controls [57]. Moreover, seronegative transplant recipients that obtained a graft from a seropositive donor had detectable levels of EBV-specific T cells in their circulation within a month after transplantation. In adult solid organ transplant recipients, class I multimers were used to compare the frequency of CD8+ T cells specific for peptides derived from EBNA3A, BMLF1, BZLF1, and LMP2 [58]. Overall, the proportion of CD8+ T cells specific for lytic and latent cycle proteins was increased in patients with chronic viral loads in the circulation compared with patients with undetectable viral load, yet similar to the levels seen in healthy controls. Moreover, the EBV-specific T cells of patients with chronic high viral load had mixed functional characteristics including the expression of the activation marker CD38 and the exhaustion marker PD-1, along with the reduced IFN-γ production. Thus, the specificity and frequency of EBV-specific T cells are similar in PTLD-free transplant patients and healthy, seropositive donors, but questions remain concerning the functional properties of these cells.
There is limited data on EBV-specific T cells in patients with PTLD. MHC class I tetramers for EBNA3A, LMP2, BZLF1, and BRLF1 were used to assess EBV-specific CD8+ T cells in the blood of PTLD patients. The frequencies and specificities of EBV-specific T cells were similar in PTLD patients to that of healthy, EBV-seropositive recipients but lower compared with transplant recipients with EBV reactivation in the absence of PTLD [59]. The total CD4+ T cell counts were lower in PTLD patients than in a control group consisting of healthy, seropositive donors, transplant patients with EBV reactivation, or primary infection and no PTLD. Clearly, additional studies are needed to determine whether differences in the frequencies of EBV-specific T cells are informative for identifying patients at risk of PTLD. Future studies that include larger cohorts of subjects, enumeration of antigen-specific CD4+ and CD8+ T cells, and the use of multimers that expand our analysis to a broader range of HLA types and EBV epitopes are necessary.
These studies most likely will need to be complemented by the analysis of the function of EBV-specific T cells since altered or impaired T cell function could also contribute to the escape and expansion of EBV-transformed B cells. Along these lines, Jones et al. [60] investigated whether CD4 or CD8 T cells from PTLD patients could mount a functional response to EBV proteins. PBMC were obtained from PTLD patients or healthy donors and stimulated in vitro for 14 days with peptides from either the latent cycle protein EBNA1 or the lytic cycle protein BZLF1, then restimulated, and the proportion of responding cells determined by flow cytometry. No differences were seen in the ability of CD8+ T cells from PTLD patients, or healthy donors, to produce IFN-γ or mobilize CD107 as a surrogate for cytotoxic function, in response to the viral antigens. In contrast, CD4+ T cells from PTLD patients had significantly reduced IFN-γ production in response to EBNA1 and BZLF1 as compared with CD4+ T cells from healthy donors. Interestingly, 10 of 14 PTLD patients carried a strain of EBV containing isoleucine in place of threonine at position 524 of EBNA. This polymorphism in EBNA1 is within a HLA-B8–presented epitope that elicits cytotoxic function by CD8+ T cells. These results suggest that variations in the EBV genome may result in alterations in the magnitude and specificity of the T cell response to the virus.
Wilsdorf et al. [61] employed a different in vitro approach to assess anti-EBV–specific T cell responses in pediatric PTLD patients by using the short-term culture of PBMC with autologous, EBV-infected B cells prior to the assessment of IFN-γ production. No differences in the proportion of T cells that produce IFN-γ were found when comparing the PTLD patients at the time of diagnosis with pediatric transplant recipients with primary EBV infection or reactivation, or with healthy controls. Nevertheless, the frequency of IFN-γ+ T cells was lower in early PTLD as compared with the late PTLD (more than 12 months post-transplant). Ning et al. [62] compared the breadth of the CD4+ and CD8+ T cell response after stimulation with a panel of lytic and latent cycle peptides by assaying the cells for simultaneous production of IFN-γ, MIP-1α TNF-α, IL-2, and CD107 mobilization. Though only two PTLD subjects were included, they showed a striking pattern with a predominantly monofunctional response such that only one cytokine or CD107 was mobilized in each cell. In contrast, healthy subjects often showed a polyfunctional response with multiple functions produced by individual cells, a property that has been associated with immune protection against viruses. Finally, a prospective study of 45 pediatric liver recipients monitored the number of EBV-specific T cells that produced IFN-γ by ELISPOT in combination with EBV viral load [63]. All seven of the children that developed PTLD had a high viral load and a low level of EBV-specific T cells. A score was established based on the number of lymphocytes per milliliter of blood, the percentage of lymphocytes in PBMC, and the number of spots per 105 PBMC in the ELISPOT. No patients with a score of greater than 2/mm3 developed PTLD. Together, these studies suggest that the combined assessment of viral load with T cell frequency and function may hold potential in identifying patients at risk of developing EBV+ PTLD.
Natural killer cells and control of EBV infection
NK cells are a rapidly responding cellular effector population of the innate immune system that is particularly important in the elimination of virally infected cells and tumor cells. Recent studies [64, 65] have revealed that NK cells play an important role in the recognition and cytotoxicity of B cells that harbor a latent EBV infection, as is seen in the EBV+ B cell lymphomas associated with PTLD. The phenotypic analysis demonstrated that NK cells in PTLD patients had predominantly CD56dimCD16− and CD56−CD16+ NK cells in contrast to asymptomatic pediatric transplant [66]. Furthermore, NK cells in PTLD patients had downregulation of the natural cytotoxicity receptor NKp46 and the activating receptor NKG2D and upregulation of PD-1, associated with cellular exhaustion, suggesting NK cells from PTLD patients are functionally impaired. Taken together, these studies indicate that further investigation of NK cell subsets may be informative in identifying PTLD-associated changes in the immune response to EBV.
B lymphoma cells
EBV+ B cell lymphomas–derived cytokines associated with PTLD
Establishment of latent EBV infection in B cells results in multiple changes in host cell gene expression that promote tumor cell survival and proliferation. One latent cycle protein, LMP1, can induce expression of host cell cytokines including IL-6 [67] and IL-10 [68]. These cellular cytokines are secreted by the infected B cells, utilized as autocrine growth factors [47, 67], and can be measured in the blood of PTLD patients. Thus, they have received interest as possible biomarkers of EBV+ B cell lymphomas in PTLD. Most studies have been done in adult transplant recipients with PTLD and have shown increases in IL-10 levels at the time of EBV+ PTLD diagnosis [69,70,71], whereas some also show increases in IL-6 at the time of diagnosis [70] and others do not [71]. IL-6 has also been shown to increase prior to the development of PTLD in adults [67] and children [72], and seems to track more closely with disease showing a rise when the disease progresses and a drop in levels with successful treatment [70]. However, elevations in IL-6 are also noted in patients with other infections or rejection [67].
Other soluble molecules produced by B cell lymphomas
CD30 is a member of the TNF receptor family and is expressed on the membrane of activated T and B cells. Soluble CD30 is released when CD30 is proteolytically cleaved from the membrane and has been detected in the circulation following viral infection, graft rejection, cancer, and autoimmunity. In a single study of 23 EBV+ PTLD patients, sCD30 was elevated during PTLD compared with transplant recipients without PTLD and healthy controls [73]. The elevations in sCD30 in PTLD patients correlated with increased EBV DNA levels. Another product of B cells, free light chain (FLC) of immunoglobulin (Ig), is also released by B cells and elevated light chains or altered kappa/lambda ratios in the circulation may be an indication of abnormal B cell activation or expansion. Elevations in FLC were noted in adult EBV+ PTLD patients but a significant proportion of matched PTLD-free transplant controls also showed elevations in FLC. Nevertheless, elevated FLC in the peripheral blood prior to EBV+ PTLD were associated with an increased risk of subsequent disease [74]. The same group conducted a study of 36 liver and hematopoietic stem cell pediatric transplant recipients, including 12 with PTLD, and analyzed for kappa and lambda FLC, monoclonal Ig (M) proteins, and sCD30 [75]. More than 90% of the samples had elevations in sCD30 and 20–30% showed elevations in kappa or lambda FLC. M proteins were found in the majority of subjects but were more frequent in the PTLD samples. Overall, these studies indicate that in transplant recipients, disturbances in B cells including elevated FLC are common. Similarly, sCD30 can be released in response to a variety of antigenic stimuli. Thus, other immune-activating events beyond EBV infection and the pathogenesis of PTLD modulate the levels of these B cell–associated markers.
Summary
Diagnosis, management, and treatment of EBV+ PTLD remain a clinical challenge. At present, there is a need for non-invasive, specific, and sensitive biomarkers that can contribute to improved clinical care. Research to identify new biomarkers has primarily focused on molecules of viral, immune, or lymphoma origin that can be detected in the blood. While there are some promising candidate molecules, there are significant challenges with identifying biomarkers that are specific indicators of risk for development of EBV+ PTLD, diagnosis, and response to treatment. The improved utility may be achieved by a combined analysis of candidate molecules with measurements of viral load. In addition, the majority of studies to date have been conducted in comparatively small numbers of adult subjects. Therefore, it will be important to establish multi-center pediatric studies to increase the study cohort size in order to evaluate and validate the most informative biomarkers.
References
Hwang CS, Macconmara M, Desai DM (2019) Pediatric abdominal organ transplantation. Surg Clin North Am 99:73–85
Swerdlow SH, Webber SA, Chadburn A, Ferry JA (2017) Post-transplant lymphoproliferative disorders, 5th edn. International Agency for Research on Cancer, Lyon
Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S (2016) Post-transplant lymphoproliferative disorders. Nat Rev Disease Primers 2:1–20
Dharnidharka V (2009) Post-transplant lymphoproliferative disease. Pediatr Nephrol 4:731–736
McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, Wyatt R, Ara M, Liereman D, Bridges N, Harmon W, CCTPT Study Group (2008) Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitors, sirolimus and steroids. Am J Transplant 5:984–989
Francis A, Johnson DW, Teixeira-Pinto A, Craig JC, Wong G (2018) Incidence and predictors of post-transplant lymphoproliferative disease after kidney transplantation during adulthood and childhood: a registry study. Nephrol Dial Transplant 33:881–889
Martinez OM, Krams SM, Lapasaran MG, Boyd SD, Bernstein D, Twist C, Weinberg K, Gratzinger D, Tan B, Armstrong B, Ikle D, Brown M, Robien M, Esquivel CO (2018) Prospective analysis of EBV+ PTLD in a multi-center study of pediatric transplant recipients. Transplantation 102:S319
Hatton O, Martinez OM, Esquivel CO (2012) Emerging therapeutic strategies for Epstein-Barr virus+ post-transplant lymphoproliferative disorder. Pediatr Transplant 16:220–229
Mucha K, Foroncewicz B, Ziarkiewicz-Wroblewska B, Krawczyk M, Lerut J, Paczek L (2010) Post-transplant lymphoproliferative disorder in view of the new WHO classification: a more rational approach to a protean disease? Nephrol Dial Transplant 25(7):2089–2098
Choquet S, Leblond V, Herbrecht R, Socie G, Stoppa AM, Vandenberghe P et al (2006) Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood 107(8):3053–3057
Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC et al (2006) Treatment of PTLD with rituximab or chemotherapy. Am J Transplant 6(3):569–576
Choquet S, Oertel S, LeBlond V, Riess H, Varoqueaux N, Dorken B et al (2007) Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol 86:599–607
Rickinson AB, Long HM, Palendira Y, Munz C, Hislop AD (2014) Cellular immune controls over Epstein Barr virus infection: new lessons from the clinic and the laboratory. Trends Immunol 35:159–169
Hislop AD, Taylor GS, Sauce D, Rickinson AB (2007) Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol 25:587–617
De Paschale M, Clerici P (2012) Serological diagnosis of Epstein Barr virus infection: problems and solutions. World J Virol 1:31–43
Fryer JF, Heath AB, Wilkinson DE, Minor PD (2011) Collaborative study to evaluate the proposed 1st WHO international standard for Epstein-Barr virus (EBV) for nucleic acid amplification technology (NAT)-based assays. World Health Organization Expert Committee on Biological Standardization. WHO/BS/11.2172. World Health Organization, Geneva
Ruf S, Behnke-Hall K, Bruhn B, Bauer J, Horn M, Beck J, Reiter A, Wagner HJ (2012) Comparison of six different specimen types for Epstein-Barr viral load quantification in peripheral blood of pediatric patients after heart transplantation or after allogeneic hematopoietic stem cell transplantation. J Clin Virol 53:186–194
Allen UD, Preiksaitis JK, the AST Infectious Diseases Community of Practice (2013) Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant 13:107–120
Gartner B, Preiksaitis JK (2010) EBV viral load detection in clinical virology. J Clin Virol 48:82–90
Hakim H, Pan J, Srivastava K, Gu Z, Bankowski MJ, Hayden RT (2007) Comparison of various blood compartments and reporting units for the detection and quantification of Epstein-Barr virus in peripheral blood. J Clin Microbiol 45:2151–2155
Tsai DE, Douglas L, Andreadis C, Vogl DT, Arnoldi S, Kotloff R, Svoboda J, Bloom RD, Olthoff KM, Brozena SC, Schuster SJ, Stadtmauer EA, Robertson ES, Wasik MA, Ahya VN (2008) EBV PCR in the diagnosis and monitoring of posttransplant lymphproliferative disorder: results of a two-arm prospective trial. Am J Transplant 8:1016–1024
Das B, Morrow R, Huang R, Fixler D (2016) Persistent Epstein–Barr viral load in Epstein Barr viral naïve pediatric transplant recipients: risk of late-onset post-transplant lymphoproliferative disease. World J Transplant 6:729–735
Orentas RJ, Schauer DW Jr, Ellis FW, Walczak J, Casper JT (2003) Monitoring andmodulation of Epstein-Barr virus loads in pediatric transplant patients. Pediatr Transplant 7:305–314
Green M, Bueno J, Towe D, Mazariegos G, Qu L, Abu-Almagd K, Reyes J (2000) Predictive negative value of persistent long Epstein Barr viral load after intestinal transplantation in children. Transplantation 70:595–596
Holman CJ, Karger AB, Mullan BD, Brundage RC, Balfour HH Jr (2012) Quantitative Epstein-Barr virus shedding and its correlation with the risk of post-transplant lymphoproliferative disorder. Clin Transpl 26:741–747
Cho Y-U, Chi H-S, Jang S, Park SH, Park C-J (2014) Pattern analysis of Epstein-Barr virus viremia and its significance in the evaluation of organ transplant patients suspected of having posttransplant lymphoproliferative disorders. Am J Clin Pathol 141:268–274
Columbini E, Guzzo I, Morolli F, Longo G, Russo C, Lombardi A, Merli P, Barzon L, Murer L, Piga S, degli Atti MLC, Locatelli F, Dello Strologo L (2017) Viral load of EBV DNAemia is a predictor of EBV-related post-transplant lymphoproliferative disorders in pediatric renal transplant recipients. Pediatr Nephrol 21:1433–1442
Parrish A, Fenchel M, Storch GA, Buller R, Mason S, Williams N, Ikle D, Conrad C, Faro A, Goldfarb S, Hayes D, Melicoff-Portillo E, Schecter M, Visner G, Sweet S, Danziger-Isakov L (2017) Epstein-Barr viral loads do not predict post-transplant lymphoproliferative disorder in pediatric lung transplant recipients: a multicenter prospective cohort study. Pediatr Transplant 21. https://doi.org/10.1111/petr.13011
Hocker B, Fickenscher H, Delecluse H-J, Bohm S, Kusters U, Schnitzler P, Pohl M, John U, Kemper MJ, Fehrenbach H, Wigger M, Holder M, Schroder M, Billing H, Fichtner A, Feneberg R, Sander A, Kopf-Shakib S, Susal C, Tonshoff B (2013) Epidemiology and morbidity of Epstein-Barr virus infection in pediatric renal transplant recipients: a multicenter, prospective study. Clin Infect Dis 56:84–92
Green M, Soltys K, Rowe DT, Webber SA, Mazareigos G (2009) Chronic high Epstein-Barr virus carriage in pediatric liver transplant recipients. Pediatr Transplant 13:319–323
Yamada M, Nguyen C, Fadakar P, Ganoza A, Human A, Shapiro R, Michaels MC, Green M (2018) Epidemiology and outcome of chronic high Epstein Barr virus pediatric kidney transplant recipients. Pediatr Transplant 22:e13147
Biomarkers Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conception framework. Clin Pharmacol Ther 69:89–95
Lo DJ, Kaplan B, Kirk AD (2014) Biomarkers for kidney transplant rejection. Nat Rev Nephrol 10:215–225
Henry NL, Hayes DF (2012) Cancer biomarkers. Mol Oncol 6:1490146
Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J et al (2004) Identification of virus-encoded microRNAs. Science 304:734–736
Hassan J, Dean J, De Gascun CF, Riordan M, Sweeney C, Connell J, Awan A (2018) Plasma EBV microRNAs in paediatric renal transplant recipients. J Nephrol 31:445–451
Navarri N, Fuligni F, Laginestra MA, Etebari M, Ambrosio MR, Sapienza M, Rossi M, De Falco G, Gibellin D, Tripodo C, Pileri SSA, Leoncini L, Piccaluga PP (2014) Molecular signature of Epstein Barr virus-positive lymphoma and post-transplant lymphoproliferative disorder suggest different roles for Epstein Barr virus. Front Microbiol. https://doi.org/10.3389/fmicb.2014.00728
Fink SEK, Gandhi MK, Nourse JP, Keane C, Jones K, Crooks P, Johrens K, Korfel A, Schmidt H, Neumann S, Tiede A, Jager U, Duhrsen U, Neuhaus R, Dreyling M, Borchert K, Sudhoff T, Riess H, Anagnostopoulos I, Trappe RU (2014) A comprehensive analysis of the cellular and EBV-specific microRNAome in primary CNS PTLD identifies different patterns among EBV-associated tumors. Am J Transplant 14:2577–2587
Kroll J, Li S, Levi M, Weinberg A (2011) Lytic and latent EBV gene expression in transplant recipients with and without post transplant lymphoproliferative disorder. J Clin Virol 52:231–235
Habib N, Buisson M, Lupo J, Agbalika F, Socie G, Germi R, Baccard M, Imbert-Marcile B-M, Dantal J, Morand P, Drouet E (2017) Lytic EBV infection investigated by detection of soluble Epstein Barr virus ZEBRA in the serum of patients with PTLD. Sci Report 7:1–9
Hopwood PA, Brooks L, Parratt R, Hunt BJ, Maria B, Alero TJ, Magdi Y, Crawford DH (2002) Persistent Epstein-Barr virus infection: unrestricted latent and lytic gene expression in healthy immunosuppressed transplant recipients. Transplantation 74:194–202
Vaysberg M, Hatton O, Lambert SL, Snow AL, Wong B, Krams SM, Martinez OM (2008) Tumor-derived variants of Epstein-Barr virus latent membrane protein 1 induce sustained Erk activation and c-Fos. J Biol Chem 283:36573–36585
De Vlaminck I, Khush KK, Strehl C, Kohli B, Neff NF, Okamoto J, Snyder TM, Weill D, Berstein D, Valantine HA, Quake SR (2013) Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 155:1178–1187
Dharnidharka VR, Ruzinova MB, Chen C-C, Parameswaran P, O’Gorman H, Goss CW, Gu H, Storch ßGA, Wylie K (2019) Metagenomic analysis of DNA viruses from posttransplant lymphoproliferative disorders. Cancer Med 8:1013–1023
Harris-Arnold A, Arnold CP, Schaffert S, Hatton O, Krams SM, Esquivel CO, Martinez OM (2015) Epstein Barr virus modulates host cell microRNA-194 to promote IL-10 production and B lymphoma cell survival. Am J Transplant 15:2813–2824
Musilova K, Mraz M (2015) MicroRNAs in B cell lymphomas: how a complex biology gets more complex. Leukemia 29:1004–1017
Beatty PR, Krams SM, Martinez OM (1997) Involvement of IL-10 in the autonomous growth of EBV transformed B cell lines. J Immunol 158:4045–4051
Zhang G, Zong J, Lin S, Berhoeven RJ, Tong S, Chen Y, Ji M, Cheng W, Tsai SW, Lung M, Pan J, Chen H (2015) Circulating Epstein Barr virus microRNAs miR-BART7 and miR-BART-13 are biomarkers for nasopharyngeal carcinoma diagnosis and treatment. Int J Cancer 136:E301–E312
Lee KT, Tank JK, Lam AK, Gan SY (2016) MicroRNAs serving as potential biomarkers and therapeutic targets in nasopharyngeal carcinoma: a critical review. Crit Rev Oncol Hematol 103:1–9
VanBuskirk AM, Malik V, Xia D, Pelletier RP (2001) A gene polymorphism associated with post-transplant lymphoproliferative disorder (PTLD) [abstract]. Transplant Proc 33:1834
Thomas R, McAulay K, Higgins C, Wilkie G, Crawford D (2005) Interferon gamma polymorphisms in posttransplant lymphoproliferative disease. Blood 106:1502–1503
Babel N, Vergopoulos A, Trappe R, Oertel S, Hammer MM, Karaianov S, Schneider N, Riess H, Papp-Vary M, Neuhaus R, Gondek L, Volk H, Reinke P (2007) Evidence for genetic susceptibility towards development of posttransplant lymphoproliferative disorder in solid organ recipients. Transplantation 85:387–391
McAuley KA, Haque T, Crawford DH (2009) Tumor necrosis factor gene polymorphism: a predictive factor for the development of post-transplant lymphoproliferative disease. Br J Cancer 101:1019–1027
Morscio J, Dierickx D, Tousseyn T (2013) Molecular pathogenesis of B cell posttranspant lymphoproliferative disorder: what do we know so far? Clin Dev Immunol 2013. https://doi.org/10.1155/2013/150835
Jagadeesh D, Woda BA, Draper J, Evens AM (2012) Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options in Oncol 13:122–136
Stojanova J, Caillard S, Rousseau A, Marquet P (2011) Post-transplant lymphoprolifeartive disease (PTLD): pharmacological, virological, and other determinants. Pharm Res 63:1–7
Falco DA, Nepomuceno RR, Krams SM, Lee PP, Davis MM, Salvatierra O, Alexander SR, Esquivel CO, Cox KL, Frankel LR, Martinez OM (2002) Identification of Epstein-Barr virus-specific CD8+ T cells lymphocytes in the circulation of pediatric transplant recipients. Transplantation 74:501–510
Macedo C, Webber SA, Donnenberg AD, Popescu I, Hua Y, Green M, Rowe D, Smith L, Brooks MM, Metes D (2011) EBV-specific CD8+ T cells from asymptomatic pediatric thoracic transplant patients carrying chronic high EBV loads display contrasting features: activated phenotype and exhausted function. J Immunol 186:5854–5862
Sebelin-Wulf K, Nguyen TD, Oertel S, Papp-Vary M, Trappe RU, Schulzki A, Pezzutto A, Riess H, Subklewe M (2007) Quantitative analysis of EBV-specific CD4/CD8 T cell numbers, absolute CD4/CD8 T cell numbers and EBV load in solid organ transplant recipients with PTLD. Transplant Immunol 17:203–2010
Jones K, Nourse JP, Morrison L, Nguyen-Van D, Moss DJ, Burrows SR, Gandhi MK (2010) Expansion of EBNA-1 specific effector T cells in posttransplantation lymphoproliferative disorders. Blood 116:2245–2252
Wilsdorf N, Eiz-Vesper B, Henke-Gendo C, Diestelhorst J, Oschlies I, Hussein K, Pape L, Baumann U, Tonshoff B, Pohl M, Hocker B, Wingen A-M, Klapper W, Kreipe H, Schulz TF, Klein C, Maecker-Kolhoff B (2013) EBV specific T cell immunity in pediatric solid organ graft recipient with posttransplantation lymphoproliferative disease. Transplantation 95:247–255
Ning RJ, Xu XQ, Chan KH, Chiang AKS (2011) Long term carriers generate Epstein-Barr virus (EBV)-specific CD4+ and CD8+ polyfunctional T-cell responses which show immunodominance hierarchies of EBV proteins. Immunol 134:161–171
Smets F, Latinne D, Bazin H, Reding R, Otte J-B, Bus J-P, Sokal EM (2002) Ratio between Epstein-Barr viral load and anti-Epstein Barr virus specific cell response as a predictive marker of posttransplant lymphoproliferative disease. Transplantation 73:1603–1610
Hatton O, Strauss-Albee DM, Zhao NQ, Haggadone MD, Pelpola JS, Krams SM, Martinez OM, Blish CA (2016) NKG2A-expressing natural killer cells dominate the response to autologous lymphoblastoid cells infected with Epstein-Barr virus. Front Immunol 7. https://doi.org/10.3389/fimmu.2016.00607
Djaoud Z, Guethlein LA, Horowitz A, Azzi T, Nemat-Gorgani N, Olive D, Nadal D, Norman PJ, Munz C, Parham P (2017) Two alternate strategies for innate immunity to Epstein Barr virus: one using NK cells and the other NK cells and γδ T cells. J Exp Med 5(214):1827–1841
Wiesmayr S, Webber SA, Macedo C, Popescu I, SmithL LJ, Metes D (2012) Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur J Immunol 42:541–550
Tosato G, Breinig MK, McWilliams HP, McKnight JL (1993) Interleukin-6 production in posttransplant lymphoproliferaive disease. J Clin Invest 91:2806–2814
Lambert SL, Martinez OM (2007) Latent membrane protein1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J Immunol 179:8225–8234
Muti G, Klersy C, Baldanti F, Granata S, Oreste P, Pezzzetti L, Gatti M, Garantini L, Caramella M, Mancini VV, Gerna G, Morra E for the Co-operative study group on PTLD (2003) Epstein-Barr virus (EBV) load and interleukin-10 in EBV-positive and EBV-negative post-transplant lymphoproliferative disorders. Br J Haematol 122:927–933
Hinrichs C, Wendlland S, Zimmermann H, Eurich D, Nauhaus R, Schlattmann P, Babel N, Riess H, Gartner B, Anagnostopoulos I, Reinke P, Trappe RU (2011) IL-6 and IL-10 in post-transplant lymphoproliferative disorders development and maintenance: a longitudinal study of cytokine plasma levels and T cell subsets in 38 patients undergoing treatment. Transpl Int 24:892–903
Baiocchi OCG, Colleoni CWB, Caballero OL, Vettore AL, Bulgarelli A, Dalbone MA, Granato CFH, Franco MF, Pestana JOM (2005) Epstein-Barr viral load, interleukin-6 and interleukin-10 levels in post-transplant lymphoproliferative disease: a nested case-control study in a renal transplant cohort. Leuk Lymphoma 46:533–539
Barton M, Wasfy S, Hebert D, Dipchaud A, Fecteau A, Grant D, Ng V, Solomon M, Chan M, Read S, Stephens D, Tellier R, Allen UD, the EBV and Associated Viruses Collaborative Group (2010) Exploring beyond viral load testing for EBV lymphoproliferation: role of serum IL-6 and IgE assays as adjunctive tests. Pediatr Transplant 14:852–858
Haque T, Chaggar T, Schafers J, Atkinson C, McAulay K, Crawford DH (2010) Soluble CD30: a serum markers for Epstein-Barr virus associated lymphoproliferative diseases. J Med Virol 83:311–316
Engels EA, Preiksaitis J, Zingone A, Landgren O (2012) Circulating antibody free light chains and risk of post-transplant lymphoproliferative disorder. Am J Transplant 12:1268–1274
Engels EA, Savoldo B, Pfeiffer RM, Costello R, Zingone A, Heslop HE, Landgren O (2013) Plasma markers of B cell activation and clonality in pediatric liver and hematopoietic stem cells transplant recipients. Transplantation 95:519–526
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.