Abstract
Acute kidney injury (AKI) is common in critically ill children and affects nearly 30–40% of patients admitted to the pediatric intensive care unit (ICU). Even with technological advances in critical care and dialysis, there is a high mortality rate of 66.8% to 90% in ICU patients. Renal replacement therapy (RRT) is often performed to treat patients with AKI. However, for optimal RRT treatment, it is crucial to consider the indications, modes of access, and prescription of each RRT method. Therefore, this review aims to discuss the various modalities of RRT in pediatric patients, which include peritoneal dialysis (PD), hemodialysis (HD), continuous RRT (CRRT), and sustained low-efficiency dialysis (SLED).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) in neonatal and pediatric patients is a significant cause of morbidity and mortality and technological advancements have led to long-term dialysis as a viable treatment option for AKI [1]. AKI is currently defined according to The Kidney Disease: Improving Global Outcomes (KDIGO) and Pediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease (pRIFLE) score based on a change in estimated creatinine clearance and urine output over a specified time [2, 3]. Common risk factors of AKI include shock, septicemia, liver disease, coagulation disorders, respiratory failure, ventilation, and vascular catheterization [4,5,6,7,8]. The worldwide incidence of pediatric AKI currently ranges from 19.3%–29.9% and has been shown to vary based on the definition used [9,10,11,12,13,14]. Mortality rates are relatively high in pediatric AKI and range from 66.8% to 90% in the ICU [6].
The current treatment for AKI patients often involves renal replacement therapy (RRT). Early initiation of RRT may prevent life-threatening complications associated with pediatric AKI and reduce mortality rates [1]. However, for optimal care of AKI patients, the indications, modes of access, and prescription of each RRT method should be considered. Therefore, this paper aims to review the various modalities of RRT utilized in neonatal and pediatric AKI patients, including peritoneal dialysis, hemodialysis, continuous RRT, and sustained low-efficiency dialysis.
Modalities of Renal Replacement Therapy
Renal replacement therapy (RRT) modalities for AKI have expanded from peritoneal dialysis (PD) and hemodialysis (HD) to continuous RRT (CRRT) and sustained low-efficiency dialysis (SLED). Advancements in use of RRT in pediatrics has led to a higher standard of care for young and critically ill patients. Since no difference in survival is seen with any dialysis method, the optimal RRT modality for children with AKI is based on the patient’s overall clinical status, on the performance of the dialytic modality, and on the availability of resources and expertise [1, 6].
HD and SLED may be performed every four to six hours, whereas PD and CRRT are ongoing daily treatments [1]. All RRT techniques involve the movement of water, solutes, and toxins across a semipermeable membrane via diffusion, convection, or a combination. In diffusion, dissolved particles flow across a semipermeable membrane down a concentration gradient, while in convection, solutes flow across a membrane with solvent drag due to a transmembrane pressure gradient (Fig. 1). A comparison of RRT modalities is shown in Table 1. Although RRT is efficacious in most patients with AKI, non-dialytic treatment strategies have also been proposed for neonates [10]. Electrolyte, protein, and glucose balance may be maintained with dietary and fluid restrictions and insulin. Fluid overload can be counter-acted with diuretics, and hypotension may be managed with dopamine. Clinicians could, therefore, contemplate these therapies in addition to considering RRT, depending on the AKI severity [8].
Peritoneal Dialysis in AKI
PD employs ultrafiltration, diffusion, and convection for blood purification [1]. It is the only modality of RRT that does not require vascular access, which is often difficult to establish in children due to their smaller blood vessel size. In addition, relatively low costs make PD the preferred treatment for pediatric AKI, especially in developing countries [1, 9]. In a worldwide survey by Raina et al., 68.5% of respondents in developing countries preferred PD for treating infant AKI while only 29.1% of physicians in developing countries and 22.2% in developed countries favored PD to treat AKI in children [9].
PD is especially useful in neonates with AKI after cardiopulmonary bypass or cardiac surgery [15, 16]. It is also indicated for AKI in neonates weighing ≤1000 g, and those at risk for hypoglycemia following fluid restriction because dialysis fluid contains dextrose [17]. In patients requiring emergent fluid removal, rapid clearance of toxins, or reversal of hyperkalemia, however, PD is not a first-line treatment, due to its lower efficiency when compared to CRRT [18].
Catheters for PD
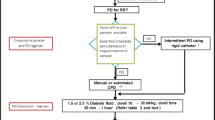
Peritoneal access is usually obtained with noncuffed acute catheters or silicone Tenckhoff catheters. A noncuffed catheter can be placed quickly at the bedside using local anesthesia [1]. It may be inserted through the linea alba (central approach) or the rectus lateralis muscle (paramedial approach) [18]. Using the Seldinger technique, the catheter is advanced into the peritoneal sheath, where it sits percutaneously. To decrease the risk of infections, the catheter exit site should face caudally [18]. Tenckhoff catheters with Dacron cuffs are surgically placed using a subcutaneous or tunneled approach. Although insertion is more invasive, these catheters have a lower risk of leakage and infection than noncuffed catheters. They are also less likely to become blocked by the omentum and are recommended for PD [18, 19]. Regardless of the type of catheter used, clinicians must decide between acute manual PD and automated PD. Manual PD allows for more precise fluid volume measurements but requires constant monitoring and adjustment, while automated PD requires less work but is not equipped to provide the small fluid volumes that neonates need [18].
PD Prescription
A prescription for PD includes dialysate type and composition; the number of exchanges; fill volume; inflow, dwell, and outflow times; and ultrafiltration volume [18]. Common commercial PD dialysate solutions can be dextrose-based with lactate as a buffer (DianealⓇ and Stay safeⓇ) or more biocompatible with a neutral pH and bicarbonate as a buffer (PhysionealⓇ and BalanceⓇ) [18]. In children with lactic acidosis, biocompatible dialysate may provide better outcomes. When filling the peritoneal cavity, dialysate volumes must be carefully titrated, so the solution does not leak from the catheter. The number of exchanges, or PD cycles, per day, depends on the patient’s fluid and electrolyte levels, but 16 to 20 exchanges in one day are the norm. For each exchange, the minimum fill volume is 200 ml/m2 for neonates and 300 ml/m2 for children, while the maximum is 800 ml/m2 and 1100 ml/m2, respectively [18]. Dialysate inflow time should be restricted to 10–15 min to preserve PD efficiency. Dwell time (15–30 min in neonates, 30–90 min in children) is longer than inflow time to maximize the amount of ultrafiltration. Effluent outflow time ranges from 20 to 30 min [1, 18]. Also, clinicians must ensure that the effluent is completely drained after each exchange to decrease the risk of abdominal and respiratory complications. Heparin (250–1000 units per liter of dialysate) and prophylactic antibiotics should be given during PD to prevent catheter-related clotting and infection. In children undergoing continuous PD, potassium (3–4 mmol/L) may need to be added to the dialysate to preserve electrolyte balance [18].
PD Efficacy and Techniques
There is no clinical consensus on the correct dose of PD in AKI. Hemodynamic stability, regression of edema, and improvement in electrolyte levels are thought to provide evidence of PD’s efficacy, however, more research must be done to support this hypothesis. There is some data on urea kinetic modeling (UKM) as a marker of dialysis efficacy in end-stage renal disease patients. UKM is expressed as a ratio of Kt/Vurea (where K = volume of dialysate drained x dialysate/plasma urea concentration, t = duration of the dialysis, and Vurea = volume of distribution of urea) [18]. In a randomized controlled trial by the Acute Renal Failure Trial Network, UKM was used to compare the efficacy of low-dose (Kt/Vurea = 2.1) vs. high-dose (Kt/Vurea = 3.88) hemodialysis in AKI patients; there was no significant difference between the two treatment doses [19]. Given this information, researchers have suggested a target of Kt/Vurea = 2.1 in AKI patients undergoing dialysis [20]. The International Society for Peritoneal Dialysis has adjusted this target for PD, stating that the recommended minimum target for small-solute clearance is a standardized weekly Kt/Vurea of 2.1 or 0.3 daily if performed 7 d per week [18, 21]. However, critically ill children have a higher metabolism, which means the target may need to be higher than 2.1 in order to facilitate adequate blood purification. To this end, certain modifications to PD have been made to improve ultrafiltration, namely continuous equilibration PD (CEPD), high volume PD (HVPD), tidal PD, and continuous flow PD (CFPD) [18]. Studies have shown that tidal PD and CFPD, particularly, improve ultrafiltration efficiency. Specifications of each method are shown in Table 2.
PD Complications
Dialysate in the abdomen increases intraperitoneal pressure, leading to hernias, back pain, and edema. Weight gain without generalized edema and genital swelling are signs of edema due to peritoneal fluid leakage [1, 18]. The obstruction of the PD catheter can also occur. Inflow obstruction is often associated with kinks in tubing or the catheter itself, whereas outflow obstruction has many more etiologies, including constipation, urinary retention causing bladder compression, omental compression of the catheter, and adhesions [18]. “Two-way” obstruction refers to intraluminal and extraluminal catheter obstruction that can occur due to blood clots and omental compression or adhesions, respectively. If an object is compressing the catheter, dialysate, or saline, then the “push-and-pull” method can be used [18]. Constipation and clots can be treated with bowel regimens and thrombolytics, respectively. If none of these interventions are successful, the catheter may need to be surgically modified or replaced with a new one. Catheter exit-site infections and peritonitis can also occur. To prevent PD-related infections, prophylactic antibiotics and training for care providers are essential [1].
Hemodialysis in AKI
HD utilizes a special filter called an artificial kidney or dialyzer to remove waste products in the blood. The blood is filtered through a semipermeable membrane via diffusion, removing smaller waste products, such as urea, creatine, potassium, and extra fluid. This method is crucial for maintenance dialysis and diseases that cause acute homeostatic imbalance (drug ingestion, hyperammonemia, and tumor lysis syndrome) [1].
Vascular Access
Vascular access is achieved through the use of a double-lumen dialysis catheter in the internal jugular, femoral or subclavian veins in acute settings. Femoral catheters are the easiest to insert but should only be used for intensive care due to the high risk of infection and thrombosis [22]. Subclavian catheters have less risk of infection but a higher risk of postoperative stenosis and procedural complications [23]. In a study, examining at dialysis by subclavian vs. internal jugular catheter, it was shown that the internal jugular catheters have a lower risk of stenosis than subclavian catheters [24]. Furthermore, right-sided internal jugular catheterization is the preferred standard as opposed to the left-side as it provides the best blood flow rates for solute clearance with the least amount of complications. The larger the access, the more blood flow occurs. However, the size of the catheter needs to be proportionate to the size of the child. In very small children, single lumen access is preferred as it is larger and provides better blood flow than a double lumen [1]. However, a single lumen catheter requires an expansion chamber to allow for pressure change, which can increase the volume of the blood circuit. Various types of catheters based on weight/size of the child are shown in Table 3. Lastly, it is recommended to change subclavian and internal jugular catheters after 3 wk and femoral catheters after 5 d in non-ICU settings to prevent infections [25]. Achieving access in neonates and children continues to be a challenge due to the small size and lack of easily visible veins.
Dialyzer
The surface area of the dialyzer must be less than or equal to the surface area of the child [1]. A large volume could be too stressful for the child and slow blood flow can lead to slower mass transfer and clotting [26]. The choice of dialyzer considers membrane biocompatibility, blood priming value, clearance and ultrafiltration characteristics [1]. The membrane can either be made from modified cellulose or a synthetic material. Synthetic membranes are the most compatible as cellulose membranes can cause activation of leukocytes and severe allergic reactions [26]. Synthetic membranes are ideal for albumin dialysis due to their high absorptive properties but may rarely cause hypotension, edema, inflammatory hyperemia, and pain. For dialyzer sterilization, steam and irradiation should be used over ethylene oxide, as it can be toxic and cause allergic reactions [26, 27]. The standard dialysate includes 138–140 mmol/L sodium, 2 mmol/L potassium, 1.25–1.75 mmol/L calcium, 0.5–1 mmol/L magnesium and 1 g/L glucose. Bicarbonate is used as a buffer and all the components are added to purified water (ultrapure preferred) by the HD machine [26]. The electrical conductivity of the solution is measured and verified by the machine before use; the temperature of the dialysate is warmed to 33.5 to 37.5 °C to compensate for heat loss.
HD Prescription
The extracorporeal blood volume is defined as 8–10% of the total blood volume and consists of the blood tubing and dialyzer priming volume. Loss of extracorporeal blood volume can cause hypotension in an anemic child requiring blood priming. Blood tubing for HD is available in 3 sizes: neonatal (25 ml), pediatric (75 ml), and adult (127 ml) [1]. Blood priming is essential for infants and young children to prevent hemoconcentration and packed red blood cells (pRBC) or 5% albumin can be used to prime the blood tubing and dialyzer. The total blood volume for neonates and children is 100 ml/kg and 80 ml/kg of body weight, respectively. A standard dialysate flow is 500 ml/min with a range of 300–800 ml/min [1, 26]. The maximum ultrafiltration rate is 0.2 ml/kg/min and depends on the target dry weight (post-dialysis weight). Underestimating dry weight may lead to hypovolemia while overestimation can lead to volume overload, causing hypertension, congestive heart failure, pulmonary edema, and left ventricular hypertrophy [1].
HD can be performed with or without an anticoagulant. In children, low blood flow rates, smaller vascular access, turbulent flow, and high hematocrit levels can increase the risk of clotting [27]. Systemic anticoagulation with unfractionated heparin can reduce clotting risk and increase circuit lifespan. Heparin is the standard anticoagulant with a pre-HD infusion of 10–20 U/kg/dose and continuous infusion of 20–30 U/kg/h [1, 26]. However, if the risk of bleeding is high in a pediatric patient, citrate anticoagulation should be used. If more citrate is being delivered than cleared, citrate accumulation (citrate lock) can occur, leading to a decrease in serum ionized calcium levels, and an increase in total calcium levels. A study by Brophy et al. showed that saline flushes can prevent the risk of citrate lock [28]. It is also best to use a bicarbonate-based solution (22 to 25 mEq/L range) and a zero-calcium bath [26]. Neither heparin nor citrate are ideal anticoagulants due to their complications. An alternate agent is prostacyclin (an inhibitor of platelet aggregation and a vasodilator), which can be metabolized rapidly and may optimize oxygen delivery and uptake. This is crucial in critically ill children with multi-organ failure. The main side effect of prostacyclin is hypotension due to vasodilation [29]. A standard pediatric HD prescription is shown in Table 4.
There are several complications of HD in infants and children. HD requires a large vessel for vascular access; a large HD catheter placement can lead to thrombosis and stenosis, causing the loss of vascular access. Hypotension is common during HD in children due to their smaller blood volume, though its risk can be reduced by slower ultrafiltration rates [26]. HD can also lead to muscle cramping and can be treated with hypertonic saline solution, glucose, or an increase in dialysate sodium concentration [1].
Continuous Renal Replacement Therapies in AKI
CRRT is the preferred modality for the management of AKI and fluid overload in critically ill children [30]. CRRT is separated into subcategories based on the method of solute clearance. It includes Continuous Veno-Venous Hemofiltration (CVVH), Continuous Veno-Venous Hemodialysis (CVVHD), and Continuous Veno-Venous Hemodiafiltration (CVVHDF). CVVH utilizes convective clearance and replacement fluid to partially/fully replace the ultrafiltrate. CVVHD utilizes a diffusive clearance by using the dialysate, while CVVHDF utilizes a combination of convective and diffusive clearance by using the dialysate and replacement fluids. Each mode is equally effective for the removal of small-molecular-weight solutes, such as urea and citrate. However, modes that utilize convective clearance (CVVH and CVVHDF) are more effective for the clearance of large-molecular-weight solutes, such as vancomycin [1]. The different modes of CRRT are shown in Fig. 2.
Schematic diagrams of commonly used continuous renal replacement therapy modalities. CVVH Continuous veno-venous hemofiltration; CVVHD Continuous veno-venous hemodialysis; CVVHDF Continuous veno-venous hemodiafiltration; RF Replacement fluid; CVVH utilizes diffusion and requires replacement fluid. CVVHD utilizes diffusion and requires dialysis fluid. CVVHD utilizes both diffusion and convection and requires dialysate and replacement solution
Vascular Access
Identifying vascular access in CRRT is similar to that in HD; quality access is proportional to the patient’s size. A short and wide catheter will provide maximum blood flow. However, a study by the Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) Registry found large catheters to have significantly longer CRRT circuit survival [31]. The use of a standard single lumen 5-French catheter in infants leads to low circuit survival [1]. Weight/size suggested catheters are shown in Table 3. The optimal site for catheter placement depends on the risks of the procedure, thrombosis, stenosis, and infection [32]. The right internal jugular vein is the preferred site due to its large caliber, direct access to superior vena cava, and lower recirculation rate. Another site is the femoral vein due to accessibility, although not ideal because of associations with higher recirculation rates and potential flow interference [31, 32].
Dialyzer
There are multiple types of hemofilters and membranes with various thicknesses, pore sizes, charges, and absorptivities that determine the extent of solute removal. The hemofilter must be proportionate to the patient’s body surface area. The preferred membrane is the AN-69 polyacrylonitrile membrane but associated with bradykinin release syndrome when used with a blood prime [33]. This event causes an abrupt drop in blood pressure shortly after CRRT initiation (5–10 min) but can be prevented by buffering the blood to physiological pH before circuit priming [1]. An alternative to AN-69 membrane is polysulfone derivative membranes, which are not associated with bradykinin release syndrome [34].
Often, infants and young patients receiving CRRT can develop hypothermia. However, hypothermia can be prevented by placing infants in radiant warmers or using heated blankets. Various CRRT machines can provide blood warmers, which prevent heat loss, or an electric heating sleeve, which can adjust the temperature of the blood (between 33 and 43 °C) [1]. Lactate-based solutions have been used previously; however, they have led to lactic acidosis, cardiac dysfunction, and hypertension. Currently, pharmacy-made bicarbonate solutions are the standard solutions used in CRRT; many of these solutions contain small amounts of lactate (3 mEq/L) for stability and physiological pH [35].
CRRT Prescription
Blood flow can be adjusted to the size of the patient if the appropriate-size catheter is placed. In pediatrics, the recommended blood flow rates are between 3 and 10 ml/kg/min and higher blood flow of 10–12 ml/kg/min in neonates and small infants when using adult-sized catheters [1, 32]. The ppCRRT Registry recommends a mean blood flow rate of 96.9 ml/min (range 10–350 ml/min) and scaled to body weight mean blood flow rate of 5 ml/kg/min (range 0.6–53.6 ml/kg/min) [36]. In critically ill children who weigh ≤15 lbs., the CRRT circuit is primed with packed red blood cells (pRBC) rather than saline to prevent excessive hemodilution and hemodynamic instability [37].
Anticoagulation is usually attained with unfractionated heparin due to its low cost and simple monitoring. But in 1–5% of pediatric patients, heparin-induced thrombocytopenia can occur with regular heparin exposure [38]. Severe bleeding has also been reported in 10–50% of cases. However, citrate-based anticoagulation can be used in patients with risk of bleeding. If more citrate is being delivered than cleared, citrate accumulation (citrate lock) can occur and saline flushes can prevent the risk of citrate lock [28]. A standard pediatric CRRT prescription is shown in Table 5.
The prescribed dose for CRRT is the effluent rate (ml/kg/h) for the clearance of small solutes. Multiple studies assessing CRRT dose and survival have illustrated no significant difference in survival when the dosage is greater than 20–25 ml/kg/h [39, 40]. However, the prescribed dose differs from the delivered dose due to circuit clotting, vascular access problems, therapy interruptions due to scheduled filter change and patient’s issues. The prescribed dosage recommended by the KDIGO criteria is an effluent volume of 20–25 ml/kg/h [41].
Sustained Low-Efficiency Dialysis in AKI
SLED is an alternative to CRRT in hemodynamically unstable AKI patients. It utilizes conventional dialysis machines with low blood pump and dialysate flow rates for ≥6 h daily [1]. SLED requires vascular access similar to CRRT and catheters with a fistula in situ are preferred. The blood flow rate should be ≤3–5 ml/kg/min for patients weighing 20–40 kg and 150 ml/min for patients >40 kg [42]. The dialysate flow rate should be ≤ twice the blood flow rate and can be slowed to 100 ml/min (6 L/h) in diffusive mode. If the extracorporeal blood volume is >10% of total blood volume, the circuit can be primed with saline/5% albumin or pRBC (if hemoglobin <7 g/dL) [42]. Lower dosage of unfractionated heparin could be used for anticoagulation but can cause bleeding. SLED can also be performed without anticoagulation; it is suggested to increase the blood flow rate by 20–25% [43]. SLED should be provided a minimum weekly Kt/Vurea of 2 and 6 compared to HD and CRRT, respectively. The standard prescription for SLED is shown in Table 6. There are some disadvantages to SLED, which include a lack of continuous dialyzing, possible loss of 15%–35% of amino acids during the process, and limited studies involving SLED in children [1].
Conclusions
AKI continues to be a prevalent illness needed to be diagnosed and treated as early as possible to maximize recovery. Children with AKI pose a challenge, especially due to their smaller size. RRT is a practical and effective treatment for pediatric AKI. In order to provide optimal care, the clinician should consider the risks and benefits of each modality and tailor therapy according to the patient’s needs. More research is needed to gather sufficient evidence to develop RRT treatment guidelines that apply to all pediatric AKI patients.
References
Sethi SK, Bunchman T, Raina R, Kher V. Unique considerations in renal replacement therapy in children: core curriculum 2014. Am J Kidney Dis. 2014;63:329–45.
Li PK, Burdmann EA, Mehta RL. World kidney day 2013: acute kidney injury—global health alert. Am J Kidney Dis. 2013;61:359–63.
Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24:191–6.
Krishnappa V, Hein W, DelloStritto D, Gupta M, Raina R. Palliative care for acute kidney injury patients in the intensive care unit. World J Nephrol. 2018;7:148–54.
Sutherland SM, Ji J, Sheikhi FH, et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8:1661–9.
Pathak S, Pandey SS, Lazarus M, Mudey A, Nagrale A. Incidences and clinical outcomes of acute kidney injury in PICU: a prospective observational study. Int J Med Health Sci. 2017;6:101–5.
Macedo E, Cerdá J, Hingorani S, et al. Recognition and management of acute kidney injury in children: the ISN 0by25 global snapshot study. PLoS One. 2018;13:e0196586.
Sethi SK, Raghunathan V, Shah S, et al. Fluid overload and renal angina index at admission are associated with worse outcomes in critically ill children. Front Pediatr. 2018;6:118.
Raina R, Chauvin AM, Bunchman T, et al. Treatment of AKI in developing and developed countries: an international survey of pediatric dialysis modalities. PLoS One. 2017;12:e0178233.
Carmody JB, Swanson JR, Rhone ET, Charlton JR. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. 2014;9:2036–43.
Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10:554–61.
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. New Engl J Med. 2017;376:11–20.
Jetton JG, Boohaker LJ, Sethi SK, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–94.
Pandey V, Kumar D, Vijayaraghavan P, Chaturvedi T, Raina R. Non-dialytic management of acute kidney injury in newborns. J Renal Inj Prev. 2016;6:1–11.
Kwiatkowski DM, Menon S, Krawczeski CD, et al. Improved outcomes with peritoneal dialysis catheter placement after cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2015;149:230–6.
Bojan M, Gioanni S, Vouhe PR, Journois D, Pouard P. Early initiation of peritoneal dialysis in neonates and infants with acute kidney injury following cardiac surgery is associated with a significant decrease in mortality. Kidney Int. 2012;82:474–81.
Alparslan C, Yavascan O, Bal A, et al. The performance of acute peritoneal dialysis treatment in neonatal period. Ren Fail. 2012;34:1015–20.
Kim YH, Resontoc LPR. Peritoneal dialysis in critically ill children. In: Deep A, Goldstein SL, editors. Critical Care Nephrology and Renal Replacement Therapy in Children. New York: SpringerLink: Springer, Cham; 2018. p. 307–23.
Palevsky PM, Zhang JH, O'Connor TZ, et al; VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20.
Paganini EP, Tapolyai M, Goormastic M, et al. Establishing a dialysis therapy/patient outcome link in intensive care unit acute dialysis for patients with acute renal failure. Am J Kidney Dis. 1996;28:581–9.
Cullis B, Abdelraheem M, Abrahams G, et al. Peritoneal dialysis for acute kidney injury. Perit Dial Int. 2014;34:494–517.
Fischbach M, Edefonti A, Schroder C, Watson A; The European Pediatric Dialysis Working Group. Hemodialysis in children: general practical guidelines. Pediatr Nephrol. 2005;20:1054–66.
Akaraborworn O. A review in emergency central venous catheterization. Chin J Traumatol. 2017;20:137–40.
Schillinger F, Schillinger D, Montagnac R, et al. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant. 1991;6:722–4.
National Kidney Foundation. KDOQI clinical practice guidelines for vascular access, 2000. Am J Kidney Dis. 2001;37:S137–81.
Preka E, Rukshana S. Haemodialysis. In: Deep A, Goldstein SL, editors. Critical Care Nephrology and Renal Replacement Therapy in Children. New York: SpringerLink: Springer, Cham; 2018. p. 271–89.
Raina R, Vijayaraghavan P, Kapur G, et al. Hemodialysis in neonates and infants: a systematic review. Semin Dial. 2017;31:289–99.
Brophy PD, Somers MJ, Baum MA, et al. Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant. 2005;20:1416–21.
Kozek-Langenecker SA, Spiss CK, Michalek-Sauberer A, Felfernig M, Zimpfer M. Effect of prostacyclin on platelets, polymorphonuclear cells, and heterotypic cell aggregation during hemofiltration. Crit Care Med. 2003;31:864–8.
Goldstein SL. Acute kidney injury in children and its potential consequences in adulthood. Blood Purif. 2012;33:131–7.
Hackbarth R, Bunchman TE, Chua AN, et al. The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the pPCRRT registry. Int J Artif Organs. 2007;30:1116–21.
Menon S, Symons JM. CRRT: technology and basic concepts. In: Deep A, Goldstein SL, editors. Critical Care Nephrology and Renal Replacement Therapy in Children. New York: SpringerLink: Springer, Cham; 2018. p. 211–21.
Bunchman TE, Brophy PD, Goldstein SL. Technical considerations for renal replacement therapy in children. Semin Nephrol. 2008;28:488–92.
Hothi DK, St George-Hyslop C, Geary D, Bohn D, Harvey E. Continuous renal replacement therapy (CRRT) in children using the AQUARIUS. Nephrol Dial Transplant. 2006;21:2296–300.
Davenport A, Will EJ, Davison AM. Hyperlactataemia and metabolic acidosis during haemofiltration using lactate-buffered fluids. Nephron. 1991;59:461–5.
Symons JM, Brophy PD, Gregory MJ, et al. Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis. 2003;41:984–9.
Eding DM, Jelsma LR, Metz CJ, Steen VS, Wincek JM. Innovative techniques to decrease blood exposure and minimize interruptions in pediatric continuous renal replacement therapy. Crit Care Nurse. 2011;31:64–71.
Verma AK, Levine M, Shalansky SJ, Carter CJ, Kelton JG. Frequency of heparin-induced thrombocytopenia in critical care patients. Pharmacotherapy. 2003;23:745–53.
Bellomo R, Cass A, Cole L, et al; RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–38.
The VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20.
Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nat Rev Nephrol. 2010;6:521–9.
Sethi SK, Sinha R, Jha P, et al. Feasibility of sustained low efficiency dialysis in critically sick pediatric patients: a multicentric retrospective study. Hemodial Int. 2018;22:228–34.
Sethi SK, Bansal SB, Khare A, et al. Heparin free dialysis in critically sick children using sustained low efficiency dialysis (SLEDD-f): a new hybrid therapy for dialysis in developing world. PLoS One. 2018;13:e0195536.
Acknowledgements
The authors thank Ms. Jennifer L. Clark, Grant/Medical Writer, Rebecca D. Considine Clinical Research Institute and Akron Children’s Hospital for her assistance in language editing. They also thank Lena Nemer for her contributions in compiling and reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
Each author has contributed equally to this article. RR will act as guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sethi, S.K., Chakraborty, R., Joshi, H. et al. Renal Replacement Therapy in Pediatric Acute Kidney Injury. Indian J Pediatr 87, 608–617 (2020). https://doi.org/10.1007/s12098-019-03150-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-019-03150-9