Abstract
Background
Obesity is a risk factor for poor transplant outcomes in the adult population. The effect of pre-transplant weight on pediatric kidney transplantation is conflicting in the existing literature.
Methods
Data was collected from the Organ Procurement and Transplantation Network (OPTN) database on recipients aged 2–21 years who received a kidney-only transplant from 1987 to 2017. Recipients were categorized into underweight, normal, overweight, and obese cohorts. Using adjusted regression models, the relationship between recipient weight and various graft outcomes (delayed graft function [DGF], acute rejection, prolonged hospitalization, graft failure, mortality) was examined.
Results
18,261 transplant recipients (mean age 14.1 ± 5.5 years) were included, of which 8.7% were underweight, 14.8% were overweight, and 15% were obese. Obesity was associated with greater odds of DGF (OR 1.3 95% CI 1.13–1.49, p < 0.001), acute rejection (OR 1.23 95% CI 1.06–1.43, p < 0.01), and prolonged hospitalization (OR 1.35 95% CI 1.17–1.54, p < 0.001) as well as greater hazard of graft failure (HR 1.13 95% CI 1.05–1.22, p = 0.001) and mortality (HR 1.19 95% CI 1.05–1.35, p < 0.01). The overweight cohort had an increased risk of graft failure (HR 1.08 95% CI 1.001–1.16, p = 0.048) and increased odds of DGF (OR 1.2 95% CI 1.04–1.38, p = 0.01) and acute rejection (OR 1.18 95% CI 1.01–1.38, p = 0.04). When stratified by age group, the increased risk was realized among younger and older age groups for obese and overweight. Underweight had lower risk of 1-year graft failure (HR 0.82 95% CI 0.71–0.94, p < 0.01), overall graft failure in the 13–17-yr. age group (HR 0.84 95% CI 0.72–0.99, p = 0.03) and acute rejection in the 2–5-yr. age group (OR 0.24 95% CI 0.09–0.66, p < 0.01).
Conclusion
Pre-transplant weight status and age impact pediatric kidney transplant outcomes. Recipient underweight status seems to be protective against adverse outcomes while overweight and obesity may lead to poorer graft and patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global epidemic of obesity and overweight is worsening. From 2011 to 2017 in the United States, the prevalence of obesity among children and adolescents aged 2 to 19 years old was 17%, affecting 12.7 million children and adolescents nationwide [1]. Obesity in children who received kidney transplants has also increased over time [2]. Obesity places children and adults at risk for various health issues including, but not limited to, diabetes mellitus and cardiovascular disease (CVD), and is also a major risk factor for mortality [3]. Compared with obesity, the prevalence of underweight status is low in developed countries [4]. Although not traditionally considered a risk factor for morbidity, underweight status increasingly has been shown to be associated with adverse health outcomes, CVD, and mortality, presumably related to malnutrition and frailty [4,5,6].
Evidence from adult and pediatric literature has shown that weight in the pre-transplant period is associated with varying kidney transplant outcomes. Generally, in the adult population, pre-transplant obesity has been associated with delayed graft function (DGF), acute rejection, and worse graft and patient survival [3, 7,8,9,10,11,12,13,14,15,16,17]. Low pre-transplant body mass index (BMI) has also been shown to be a risk factor for poorer kidney transplant outcomes [15]. Findings from a limited number of pediatric studies are conflicting with some demonstrating a relationship between obesity and poor outcome, but others finding no significant relationship [2, 18, 19]. Using North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) data, Hanevold et al. described that age modified the association of mortality with obesity [2]. Adverse outcomes within the overweight and underweight populations are poorly characterized in the pediatric population.
The purpose of this study was to take a more granular approach towards the examination of pre-transplant weight status of a large pediatric population on kidney transplant outcomes. We hypothesized that transplant outcomes such as graft failure, mortality, DGF, acute rejection, and prolonged hospitalization would be worse in children classified as underweight, overweight, and obese pre-transplant compared to normal weight children and risk would differ by age.
Methods
Setting and participants
The Organ Procurement and Transplantation Network (OPTN) database is a registry of all solid organ transplants performed nationwide which include transplant information that is collected pre- and post-transplant, at 6 months, 1 year, and then annually. The OPTN database was current as of April, 2018 [20]. Transplant recipients were followed from the date of transplant until allograft failure, death, or the end of the study period. Children and adolescents, aged 2–21 years, who received a kidney transplant from January 1987 through December 2017 were identified and included in the analysis. Young adults aged 18–21 years were included in the study to comprise a population more representative of clinical practice at pediatric transplant centers. Higher risk populations with a history of previous transplant, multi-organ transplantation, and diabetes mellitus at the time of transplant or previous malignancy were excluded. Those without a documented pre-transplant weight and height were excluded as well. The final study population included 18,261 pediatric kidney transplant recipients (Fig. 1). The study did not require review by the Institutional Review Board of Northwell Health as it did not meet criteria for human subject research.
Demographic and clinical variables
Collected demographic data included recipient age, sex, height, weight, BMI, race, donor age, and donor type (living or deceased). Transplant recipients were categorized into the following age groups: 2–5 years, 6–12 years, 13–17 years, and 18–21 years. Age- and sex-specific z-scores were generated for BMI. Clinical covariates included primary diagnosis, transplant year, cold ischemic time, peak panel reactive antibody (PRA), donation after cardiac death (DCD), prior dialysis, dialysis duration, human leukocyte antigen (HLA) mismatch, graft survival time, induction therapy, and immunosuppressant medications. Insurance status (private versus public health insurance) was used as a surrogate for socioeconomic status.
Pre-transplant weight status was the primary variable of interest. This was categorized as underweight, normal weight, overweight, or obese. This classification is based on the Centers for Disease Control and Prevention (CDC) BMI-for-age percentile growth charts for recipients ≤ 19 years old: underweight < 5th percentile; normal weight 5th percentile to < 85th percentile; overweight 85th to < 95th percentile; and obese ≥ 95th percentile. For recipients 20–21 years old, weight status was categorized by BMI (kg/m2): underweight < 18.5; normal weight 18.5 to 24.9; overweight 25 to 29.9; and obese ≥ 30 [21]. Weight and height at the time of transplant were used to calculate BMI.
Outcomes
The primary endpoints of the study were death-censored graft failure and mortality. One year death-censored graft failure was also analyzed. Due to large amounts of missing data, the causes of graft failure and mortality data were not analyzed. Secondary outcomes included DGF, acute rejection, and prolonged hospitalization. DGF was defined based on the need for dialysis within the first week of transplantation. Acute rejection reported within 1 year of transplant was considered. Due to unavailable time to rejection data, analysis was limited to patients who received a minimum follow-up time of 1 year from transplant for acute rejection analysis. Prolonged hospitalization was defined as post-transplant inpatient stay of over 14 days.
Statistical analysis
Chi-square tests and analysis of variance (ANOVA), with post-hoc z-test (Bonferroni correction) and Tukey tests, were used to compare the demographic and clinical characteristics among the different weight categories. Regression analyses were performed to determine the effect of pre-transplant weight status on transplant outcomes. Using multiple logistic regression, odds ratios (OR) were generated for DGF, acute rejection, and prolonged hospitalization. Cox proportional hazards regression and survival plots with life tables were used to examine death censored-graft failure and mortality. Transplant recipients were analyzed as a whole and then stratified by age group.
Regression models were adjusted for covariates that were selected a priori after conducting a literature review and included variables with < 15% missing data. The adjusted model (covariate, % missing) included pre-transplant weight status along with age, sex, race, insurance status (12.8%), transplant year, donor type, donor age, HLA mismatch (0.7%), cold ischemia time (11.5%), primary diagnosis (2.5%), history of prior dialysis (0.7%), and dialysis duration. Age was not included in models that were stratified by age group. Normal weight status was used as the reference group for all models.
To account for missing data in regression analyses, multiple imputations were run for the covariates (not for the outcomes) using the Markov Chain Monte Carlo method. Tests for multicollinearity and interactions between covariates were performed. Statistically significant data had a p value of < 0.05 and two-sided tests of hypotheses were used. For data analyses, SPSS 25.0 (IBM SPSS, Armonk, NY, USA) statistical software was utilized.
Results
Demographic and clinical data
Data were collected on 18,261 transplant recipients, 2745 (15%) of which were obese. Among the different weight categories, significant differences were apparent in all demographic and clinical factors with the exception of dialysis duration (Table 1). In post-hoc analysis, the overweight and obese groups were significantly younger than the normal weight group, whereas the underweight group was significantly older than the normal weight group. Compared to the normal weight group, the overweight and obese groups were more frequently male and of black race. The following primary diagnoses differed among weight groups: obstructive uropathy, cystinosis, systemic lupus erythematosus, dysplasia/agenesis, sickle cell, Alport syndrome, Prune Belly, chronic glomerulopathy, and HIV nephropathy. The underweight group had longer follow-up and cold ischemia time compared with the other weight groups. Dialysis was more common in the underweight group compared with obese group. Donors were younger and living donors were less frequent in the obese group compared with normal weight. Post-hoc analysis was not done for variables with large amounts of missing data. Induction and immunosuppression medications had large amounts of missing data (Supplementary Table 1).
Graft failure
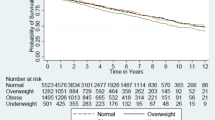
Over the study period, 7411 (40.6%) transplant recipients had failed grafts. By weight status, 42.1% (N = 672), 39.9% (N = 4473), 41.1% (N = 1114), and 42% (N = 1152) of underweight, normal weight, overweight, and obese renal transplant recipients, respectively, experienced graft failure. The incidence of death-censored graft failure among the different cohorts was measured using adjusted survival plots (Fig. 2). In the adjusted model, overweight (HR 1.08 95% CI 1.001–1.16, p = 0.048) and obese (HR 1.13 95% CI 1.05–1.22, p = 0.001) cohorts were more likely to have failed grafts than the normal weight cohort. The underweight group trended towards a lower hazard of graft failure (HR 0.91 95% CI 0.83–1.001, p = 0.05). When examining 1-year graft failure, overweight (HR 1.16 95% CI 1.05–1.28, p < 0.01) and obese (HR 1.17 95% CI 1.07–1.29, p = 0.001) were associated with an increased hazard of graft failure while underweight (HR 0.82 95% CI 0.71–0.94, p < 0.01) was associated with a lower hazard of graft failure.
Survival plot and life table for allograft survival. The graft failure rate for all four weight categories was graphed as a function of patient time in days adjusted for recipient’s sex and age, recipient’s race, diagnosis, insurance status, transplant year, donor type, donor age, human leukocyte antigen (HLA) mismatch, cold ischemia time, history of prior dialysis and duration of dialysis
Differences by age group and weight status were detected for graft failure (Table 2). Among the 12–17-year age group, when compared to normal weight, underweight was associated with a lower hazard of graft failure (HR 0.84 95% CI 0.72–0.99, p = 0.03) whereas overweight (HR 1.19 95% CI 0.05–1.35, p < 0.01) and obese (HR 1.28 95% CI 1.14–1.45, p < 0.001) were associated with a greater hazard of graft failure. There was a greater hazard of graft failure among the 18–21-year age group as well (HR 1.38 95% CI 1.21–1.58, p < 0.001).
Mortality
1907 recipients (12.3% of underweight, 10.1% of normal weight, 10.5% of overweight, and 10.9% of obese) died during the study period. A survival plot was created to approximate the probability of death over time for the different weight cohorts (Fig. 3). Obese recipients had a 1.19 times greater hazard of mortality when compared with normal weight recipients (95% CI 1.05–1.35, p < 0.01). Underweight and overweight statuses were not associated with mortality.
Survival plot and life table for patient survival. The recipient survival rate for all four weight categories was graphed as a function of patient time in years, from the recipient’s day of transplant to last follow-up or death adjusted for recipient’s sex and age, recipient’s race, diagnosis, insurance status, transplant year, donor type, donor age, human leukocyte antigen (HLA) mismatch, cold ischemia time, history of prior dialysis and duration of dialysis
When examining mortality by age group, overweight (HR 1.24 95% CI 1.02–1.51, p < 0.03) and obesity (HR 1.58 95% CI 1.26–1.96, p < 0.001) were associated with a greater hazard of mortality in the 18–21-year age group. Obesity (HR 1.5 95% CI 1.03–2.18, p = 0.03) was also associated with increased mortality in the 2–5-year age group; however, overweight (HR 0.5 95% CI 0.26–0.95, p = 0.04) was actually protective against mortality (Table 2).
Delayed graft function
A total of 1749 out of 18,178 (9.6%) recipients experienced DGF (0.5% missing). The distribution by weight status was: 9.6% (N = 153) for underweight patients, 9% (N = 1006) for normal weight patients, 10.5% (N = 286) for overweight patients and 11.1% (N = 304) for obese patients. The overweight and obese weight categories were significantly associated with a greater likelihood of DGF in the adjusted model (OR 1.2 95% CI 1.04–1.38, p = 0.01 for overweight; OR 1.3 95% CI 1.13–1.49, p < 0.001 for obese). There was no association found with the underweight category. When stratified by age group, overweight and obesity were only associated with delayed graft function in the 18–21-year-old age group, p < 0.05 (Table 3).
Acute rejection
A total of 3088 out of 12,796 (24.1%) recipients experienced acute rejection within 1 year of transplant (21.2% missing). The distribution among underweight, normal weight, overweight, and obese patients was 260 (24.6%), 1867 (23.6%), 483 (25.1%), and 478 (25.1%), respectively. Among the entire group, the overweight (OR 1.18 95% CI 1.01–1.38, p = 0.04) and obese (OR 1.23 95% CI 1.06–1.43, p < 0.01) groups were associated with a greater likelihood of acute rejection in the adjusted model. However, when examined by age strata, overweight and obese recipients were not more likely to experience acute rejection compared to normal weight individuals (Table 3). An inverse association between the underweight group and acute rejection emerged in the 2–5-year-old age group.
Prolonged hospitalization
Among the cohort, 1656 out of 13,123 (12.6%) recipients had a prolonged hospital stay (28.1% missing). Of these, 107 (11.1%), 952 (11.8%), 265 (13.2%), 332 (15.7%) were underweight, normal weight, overweight, and obese, respectively. Underweight and overweight statuses were not associated with prolonged hospitalization. However, there was an association between obesity and greater likelihood of prolonged hospitalization (OR 1.35 95% CI 1.17–1.54, p < 0.001). With age stratification, obesity was associated with a 1.78 times increased odds of prolonged hospitalization in the 2–5-year-old age group (95% CI 1.38–2.29, p < 0.0001).
Discussion
This analysis of a national registry of pediatric kidney transplant recipients demonstrated significant differences in transplant outcomes among recipients in different weight cohorts; the effect of which was found to be modified by age. Obese recipients were found to be at higher risk of mortality and graft failure compared to normal weight recipients. Additionally, those with obese weight status were more inclined to experience DGF, acute rejection, and prolonged hospitalization. Similarly, when compared to normal weight recipients, the overweight cohort was at greater risk of graft failure, acute rejection, and delayed graft function. After age stratification, these outcome differences remained significant in younger and older age groups. Surprisingly, underweight status was found to be protective for 1-year graft failure, graft failure in the 13–17-year age group, and acute rejection in the 2–5-year age group.
Pre-transplant weight status is an important consideration in transplantation given what is known about post-transplant weight gain. Studies in adults and children have demonstrated that obesity post-transplant is common and that pre-transplant elevated BMI is a predictor of weight gain after transplantation [22, 23]. Mitsnefes et al. reported in a small study of children that the rate of obesity doubled in the first year post-transplant [23]. Factors that may contribute to pre- and post-transplant weight gain include reduced exercise capacity due to chronic kidney disease-related fatigue and reduced muscle strength, the metabolic effect of steroids and other medications, and sedentary lifestyle [24]. Although children are also at risk for cardiovascular disease after transplant, there may be other factors related to obesity (e.g., inflammation, poor wound healing, pro-thrombotic state, technical complications, drug pharmacokinetics) [25,26,27] that lead to worse outcomes in children that have received kidney transplants. Furthermore, children with obesity experience higher workload within the nephrons which culminates in glomerular hyperfiltration, a non-immunologic contributor to chronic allograft nephropathy, and in turn, graft loss [28]. With regard to underweight status, it is thought that underweight patients may be at greater risk for cardiovascular diseases post-transplant due to chronic inflammation or malnutrition [9]. Some have also proposed that higher infection rates in underweight patients may lead to an increase in the incidence of chronic allograft nephropathy and graft failure [9]. Considering these factors, a recent study from the US Renal Data Study (USRDS) has shown that pre-transplant weight status may affect transplantation rates. Ku et al. demonstrated that obese and underweight children on dialysis were less likely to receive a kidney transplant compared to normal weight children [29].
Overall, the results of our study are consistent with a growing body of adult literature showing the detrimental effects of obesity on allograft outcomes [30,31,32]. Our study adds to existing literature by describing the different risk profiles that underweight, overweight, and obese statuses confer on transplant outcomes in children. In a recent publication, Dick et al. found no difference in mortality, graft survival, or DGF among children < 18 years classified as severely thin, normal weight, and severely obese using World Health Organization (WHO) criteria [18]. Similar to our results, Winnicki et al. showed that obese recipients have an increased risk of graft failure compared with normal weight. However, both of these studies found no differences in DGF or acute rejection and no differences in outcome for overweight and underweight categories. Winnicki et al. demonstrated a trend towards improved outcomes in underweight patients, but their analysis did not evaluate acute rejection or DGF as outcomes in adjusted models. Our study population differs from these recent reports in that it examined children and adolescents from age 2–21 years and excluded known high-risk groups. Dick et al. included children < 2 years and both studies did not exclude other high-risk groups. Dick et al. also used the extreme of weights (severe thin and obese) compared to a reference group that included, by definition, underweight, overweight, and obese children. Additionally, different covariates were used in these studies and neither study examined death-censored graft failure, which may explain the conflicting results between these reports.
This study also highlighted that age group modified the effect of pre-transplant weight on transplant outcomes. An older pediatric study using NAPRTCS data from 1987 to 2002 found no discrepancy in overall mortality, graft survival, or acute rejection between pre-transplant obese and non-obese children; however, differences were found when stratified by age group [2]. When examining children aged 6–12 years, Hanevold et al. found a higher risk of death in obese compared to non-obese children, which differed from our results of higher risk in the lowest and highest age groups for overweight and obese groups. Similar to Hanevold et al., we found that there was a higher risk of graft failure in obese children in the 13–17-year age group, in addition to the overweight group [2]. This difference could be explained by era-effect or by stratification of the study population into four groups with normal weight range as the control group as opposed to a comparison of obese to non-obese patients. Lastly, these results demonstrated that an obese cohort has a higher risk of DGF in the 18–21-year group and prolonged hospitalization in the 2–5-year group, which has not been reported in any previous pediatric study. A possible explanation for the greater DGF risk in the older age group could be due to poorer quality allografts (e.g., more acute tubular necrosis) that is exacerbated by overweight and obese weight compared to those < 18 years who are allocated higher quality kidneys.
Contrary to our stated hypothesis, we found that pre-transplant underweight status was significantly protective for 1-year graft failure, overall graft failure in the 13–17-year age group, and acute rejection in the 2–5-year age group. Although the reasons why pre-transplant underweight status would be protective in the pediatric population are not readily apparent, a possible explanation could be related to the differences in bioavailability of immunosuppressant drugs by weight. It may be easier to target trough levels in underweight individuals thus leading to better outcomes; however, this may not explain it in entirety as these patients may no longer be underweight in the post-transplant period due to weight gain [27]. Furthermore, the reasons why certain age groups of underweight status would be protective over other age groups are not clear, but compliance may be better in the younger age group. Adult studies have shown that underweight patients, in general, suffer from worse transplant outcomes including lower graft survival, increased mortality, prolonged hospitalization, and increased delayed graft function [9, 11, 15, 29]. Chang et al. found adult underweight patients to be more than two times at hazard for death (HR 2.13 95% CI = 1.09–1.54) [9]. Conversely, Meier-Kriesche et al. found lower BMI to be protective for delayed graft function [15]. Gore et al. supplemented their data by adjusting for obesity-related covariates, which showed that underweight was associated with lower incidence of DGF as well [11]. Underweight status in adults may confer different health outcomes compared to children as underweight may be a surrogate for frailty in the adult population.
This study contains some important limitations. Over the past 30 years, immunosuppression regimens, infection prevention, and monitoring strategies have changed vastly; therefore, even though adjustments for transplant year were made, generalizing transplant outcomes over this period by pre-transplant weight has its limitations. Given that this was a retrospective study of a large database, variation in covariates throughout the period before and after transplantation could not be accounted for. Residual confounding from unmeasured confounders/modifiers (e.g., hypertension, growth hormone use, or nutritional status) that could account for the underlying differences in transplant outcomes may have been present. Additionally, the OPTN dataset has missing data that may have influenced results. Potentially important variables such as induction and maintenance immunotherapy and PRA were not included in these models. Outcome variables of acute rejection and prolonged hospitalization also had missing data. Another limitation is the inclusion of pre-emptively transplanted recipients. In these recipients, much of their weight could be reflective of hypervolemia and therefore confound results. Potentially, if these recipients had excess fluid removed, they would be in a different weight category. Somewhat similarly, in dialyzed patients, the study was not able to account for the difference between “dry weight” and weight at transplant, which may significantly differ. Lastly, the patient’s health insurance was used as a surrogate marker for socioeconomic status but due to its multifarious manner, this does not fully encompass the patient’s socioeconomic status. Despite these limitations, this study represents the largest series of patients looking at pediatric transplant weight as a risk for many different outcomes.
In conclusion, the results of this study highlight that pre-transplant weight status among pediatric kidney transplant recipients is significantly associated with transplant outcomes. Furthermore, the risk conferred by weight status is modified by age group. Overall, pediatric transplant recipients with BMIs in the obese range are more likely to experience DGF, acute rejection prolonged hospitalization, and are at a higher risk for graft failure and death. Underweight pre-transplant status appears to be protective for certain age groups. Obese and overweight pediatric patients should be counseled to lose weight prior to transplantation; however, the risks of delaying transplant for weight loss (i.e., longer exposure to dialysis) need to be taken into consideration. Eligibility for transplant and defining risk categories based on pre-transplant weight status should be further explored by the transplant community.
References
Ogden CL, Carroll MD, Flegal KM (2015) Prevalence of obesity among adults and youth: United States, 2011-2014. National Center for Health Statistics, Hyattsville, MD
Hanevold CD, Ho PL, Talley L, Mitsnefes MM (2005) Obesity and renal transplant outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 115:352–356
Pi-Sunyer FX (1991) Health implications of obesity. Am J Clin Nutr 53:1595S–1603S
Park D, Lee JH, Han S (2017) Underweight: another risk factor for cardiovascular disease?: a cross-sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine 96:e8769
Vestberg D, Rosengren A, Eeg-Olofsson K, Miftaraj M, Franzen S, Svensson AM, Lind M (2018) Body mass index as a risk factor for coronary events and mortality in patients with type 1 diabetes. Open Heart 5:e000727
Wake M, Clifford SA, Patton GC, Waters E, Williams J, Canterford L, Carlin JB (2013) Morbidity patterns among the underweight, overweight and obese between 2 and 18 years: population-based cross-sectional analyses. Int J Obes 37:86–93
Armstrong KA, Campbell SB, Hawley CM, Johnson DW, Isbel NM (2005) Impact of obesity on renal transplant outcomes. Nephrology 10:405–413
Baum CL (2001) Weight gain and cardiovascular risk after organ transplantation. JPEN J Parenter Enteral Nutr 25:114–119
Chang SH, Coates PT, McDonald SP (2007) Effects of body mass index at transplant on outcomes of kidney transplantation. Transplantation 84:981–987
Glanton CW, Kao TC, Cruess D, Agodoa LY, Abbott KC (2003) Impact of renal transplantation on survival in end-stage renal disease patients with elevated body mass index. Kidney Int 63:647–653
Gore JL, Pham PT, Danovitch GM, Wilkinson AH, Rosenthal JT, Lipshutz GS, Singer JS (2006) Obesity and outcome following renal transplantation. Am J Transplant 6:357–363
Howard RJ, Thai VB, Patton PR, Hemming AW, Reed AI, Van der Werf WJ, Fujita S, Karlix JL, Scornik JC (2002) Obesity does not portend a bad outcome for kidney transplant recipients. Transplantation 73:53–55
Johnson DW, Isbel NM, Brown AM, Kay TD, Franzen K, Hawley CM, Campbell SB, Wall D, Griffin A, Nicol DL (2002) The effect of obesity on renal transplant outcomes. Transplantation 74:675–681
Lafranca JA, IJ JN, Betjes MG, Dor FJ (2015) Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med 13:111
Meier-Kriesche HU, Arndorfer JA, Kaplan B (2002) The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation 73:70–74
Nicoletto BB, Fonseca NK, Manfro RC, Goncalves LF, Leitao CB, Souza GC (2014) Effects of obesity on kidney transplantation outcomes: a systematic review and meta-analysis. Transplantation 98:167–176
Sancho A, Avila A, Gavela E, Beltran S, Fernandez-Najera JE, Molina P, Crespo JF, Pallardo LM (2007) Effect of overweight on kidney transplantation outcome. Transplant Proc 39:2202–2204
Dick AAS, Hansen RN, Montenovo MI, Healey PJ, Smith JM (2017) Body mass index as a predictor of outcomes among pediatric kidney transplant recipient. Pediatr Transplant. https://doi.org/10.1111/petr.12992
Winnicki E, Dharmar M, Tancredi DJ, Nguyen S, Butani L (2018) Effect of BMI on allograft function and survival in pediatric renal transplant recipients. Pediatr Nephrol 33:1429–1435
Organ Procurement & Transplantation Network (2018) Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA; Data as of April, 2018
(May 30, 2000) Centers for Disease Control and Prevention. CDC growth charts: United States http://www.cdc.gov/growthcharts/
Baum CL, Thielke K, Westin E, Kogan E, Cicalese L, Benedetti E (2002) Predictors of weight gain and cardiovascular risk in a cohort of racially diverse kidney transplant recipients. Nutrition 18:139–146
Mitsnefes MM, Khoury P, McEnery PT (2002) Body mass index and allograft function in pediatric renal transplantation. Pediatr Nephrol 17:535–539
Clapp EL, Bevington A, Smith AC (2012) Exercise for children with chronic kidney disease and end-stage renal disease. Pediatr Nephrol 27:165–172
Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR, American Heart Association Atherosclerosis H, Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young CoNPA, Metabolism, Council on Clinical C (2013) Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 128:1689–1712
Pinto H, Leal R, Rodrigues L, Santos L, Romaozinho C, Macario F, Alves R, Bastos C, Roseiro A, Costa F, Campos M, Mota A, Figueiredo A (2017) Surgical complications in early post-transplant kidney recipients. Transplant Proc 49:821–823
Kaplan B, Gaston RS, Meier-Kriesche HU, Bloom RD, Shaw LM (2010) Mycophenolic acid exposure in high- and low-weight renal transplant patients after dosing with mycophenolate mofetil in the Opticept trial. Ther Drug Monit 32:224–227
Ladhani M, Lade S, Alexander SI, Baur LA, Clayton PA, McDonald S, Craig JC, Wong G (2017) Obesity in pediatric kidney transplant recipients and the risks of acute rejection, graft loss and death. Pediatr Nephrol 32:1443–1450
Ku E, Glidden DV, Hsu CY, Portale AA, Grimes B, Johansen KL (2016) Association of body mass index with patient-centered outcomes in children with ESRD. J Am Soc Nephrol 27:551–558
Naik AS, Sakhuja A, Cibrik DM, Ojo AO, Samaniego-Picota MD, Lentine KL (2016) The impact of obesity on allograft failure after kidney transplantation: a competing risks analysis. Transplantation 100:1963–1969
Hill CJ, Courtney AE, Cardwell CR, Maxwell AP, Lucarelli G, Veroux M, Furriel F, Cannon RM, Hoogeveen EK, Doshi M, McCaughan JA (2015) Recipient obesity and outcomes after kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant 30:1403–1411
Kwan JM, Hajjiri Z, Metwally A, Finn PW, Perkins DL (2016) Effect of the obesity epidemic on kidney transplantation: obesity is independent of diabetes as a risk factor for adverse renal transplant outcomes. PLoS One 11
Acknowledgements
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
K.K contributed to data analysis and writing of the manuscript. D.J. contributed to study design, performance of the research, data analysis, and writing of the manuscript. C.S. contributed to the study design, data analysis, writing of the manuscript, and supervision of the research. P.S., R.F., L.I., L.C, L.T, E.M, E.G., and A.F. contributed to the study concept and writing of the manuscript.
Corresponding author
Ethics declarations
The study did not require review by the Institutional Review Board of Northwell Health as it did not meet criteria for human subject research.
Conflict of interest
The authors report no conflicts of interest.
Additional information
Kiranjot Kaur and Daniel Jun are Co-first Authors
Electronic supplementary material
ESM 1
(DOCX 64 kb)
Rights and permissions
About this article
Cite this article
Kaur, K., Jun, D., Grodstein, E. et al. Outcomes of underweight, overweight, and obese pediatric kidney transplant recipients. Pediatr Nephrol 33, 2353–2362 (2018). https://doi.org/10.1007/s00467-018-4038-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4038-8