Abstract
Background
Obesity is prevalent in children with chronic kidney disease (CKD), but the health consequences of this combination of comorbidities are uncertain. The aim of this study was to evaluate the impact of obesity on the outcomes of children following kidney transplantation.
Methods
Using data from the ANZDATA Registry (1994–2013), we assessed the association between age-appropriate body mass index (BMI) at the time of transplantation and the subsequent development of acute rejection (within the first 6 months), graft loss and death using adjusted Cox proportional hazards models.
Results
Included in our analysis were 750 children ranging in age from 2 to 18 (median age 12) years with a total of 6597 person-years of follow-up (median follow-up 8.4 years). Overall, at transplantation 129 (17.2%) children were classified as being overweight and 61 (8.1%) as being obese. Of the 750 children, 102 (16.2%) experienced acute rejection within the first 6 months of transplantation, 235 (31.3%) lost their allograft and 53 (7.1%) died. Compared to children with normal BMI, the adjusted hazard ratios (HR) for graft loss in children who were underweight, overweight or diagnosed as obese were 1.05 [95% confidence interval (CI) 0.70–1.60], 1.03 (95% CI 0.71–1.49) and 1.61 (95% CI 1.05–2.47), respectively. There was no statistically significant association between BMI and acute rejection [underweight: HR 1.07, 95% CI 0.54–2.09; overweight: HR 1.42, 95% CI 0.86–2.34; obese: HR 1.83, 95% CI 0.95–3.51) or patient survival (underweight: HR 1.18, 95% CI 0.54–2.58, overweight: HR 0.85, 95% CI 0.38–1.92; obese: HR 0.80, 95% CI 0.25–2.61).
Conclusions
Over 10 years of follow-up, pediatric transplant recipients diagnosed with obesity have a substantially increased risk of allograft failure but not acute rejection of the graft or death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of childhood obesity is increasing worldwide. In Australia, in 2011–2012, over 18 and 7% of children (aged 2–17 years) were either overweight or obese, respectively; the prevalence of childhood obesity was even higher among children of a similar age (2–19 years) in the USA (17%) [1, 2]. The prevalence of overweight and obesity has also risen in developing countries, from 8% in 1980 to about 13% by 2013, and in both boys and girls [3]. Obesity is associated with chronic kidney disease (CKD) and may also predispose to a broad range of adverse health outcomes in children with CKD, such as diabetes, hypertension and cardiovascular disease [4, 5].
Obesity is particularly common in children who have received a kidney transplant [6]. The overall prevalence of childhood obesity in those with CKD Stage 4 to 5D has been reported to range from 8 to 13% [7–9], increasing to 17% at 6 months after transplantation and to 29% at 12 months post transplantation [10, 11]. The causal pathways for obesity in this setting are complex, but they can in part be attributed to the appetite-stimulating effects of some immunosuppressive agents, such as high-dose steroids [12].
Obesity is a potential risk factor for delayed graft function, graft loss and cardiovascular and all-cause mortality in adult transplant recipients [13, 14], but the evidence supporting a similar relationship is less clear in the pediatric transplant population. Among 2- to 17-year-olds from the North American Pediatric Renal Transplant Cooperative Study there did not appear to be an increased risk of graft failure or death in transplant recipients with obesity [8], but an increased risk of death in a post-hoc sub-group of 6- to 12-year-old children was found. It is possible that the loss to follow-up among adolescents in this register masked greater effects. Thus, the aim of our study was to evaluate the associations between obesity and acute rejection, graft loss and patient survival in pediatric transplant recipients.
Materials and methods
Study population
Using data from the Australian and New Zealand Dialysis and Transplant (ANZDATA) Registry, we included in our study all children aged 2–18 years of age who had received their first kidney transplant between 1 January1994 and 31 December 2013. Data on the outcome of acute rejection had only been continuously compiled in the ANZDATA Registry from 1 April 1997 onward, and so only recipients who were transplanted after this date were included in our analysis. The ANZDATA Registry is a comprehensive database of all children and adults who have received renal replacement therapy since 1965 in Australia and New Zealand. Recipients aged <2 years were excluded from the analysis as body mass index (BMI) standards were not available for this age group [15].
Measurement of the exposure
The BMI was calculated as weight (in kilograms) on the day of transplantation divided by height (in meters) squared. Height was recorded on the day of transplantation or within 60 days of the transplantation. If these data were not available, then height was interpolated from the last pre-transplant and earliest post-transplant heights. The unadjusted BMI was then transformed into BMI for age and sex Z-scores using the Centers for Disease Control and Prevention Growth reference charts [15]. BMI was also categorized using Z-scores corresponding to BMI at 18 years of age into the categories of underweight (included all grades of thinness; BMI < 18.5), normal weight (BMI 18.5 to ≤25), overweight (BMI 25 to ≤30) and obesity (BMI > 30), as recommended by the Childhood Obesity Working Group of the International Obesity Taskforce [16]. We analyzed BMI as a linear and categorical variable in all models. In none of the models was the test for trend significant. Linearity between BMI and all outcomes was also assessed using restricted cubic splines. Adding a spline term did not improve the model fit for acute rejection (p = 0.3), graft failure (p = 0.2) or patient survival (p = 0.8); accordingly, only categorical data are presented here.
Covariates of interest
Sex, ethnicity (categorized as Caucasian, indigenous and other), age at first renal replacement therapy and age at transplantation, era of transplant (categorized into four 5-year periods), primary renal disease (categorized into glomerulonephritis, cystic, urological and other), type of renal replacement therapy, donor source (categorized into living and deceased), age and sex of donor, human leukocyte antigen (HLA) mismatches, total ischemic time (from cross-clamp to perfusion to the nearest hour) and panel reactive antibodies (PRA) were all analyzed as potential covariates. Total ischemic time was used, as the ANZDATA Registry does not collect cold and warm ischemic times separately.
Outcomes of interest
The primary outcome was all-cause mortality. Causes of death were reported as cardiac, vascular, infection, malignancy, withdrawal and other. The two secondary outcomes were overall and death-censored graft survival, and acute rejection (within 6 months of transplantation). Causes of graft loss were reported as chronic allograft nephropathy, vascular, technical, glomerulonephritis, non-adherence, death with function and other. Time to first event was ascertained and reported for all outcomes. All graft outcomes, including causes of graft loss and acute rejection, were as reported by the treating nephrologist.
Statistical analyses
The baseline characteristics of the study cohort were expressed as the number and proportion of recipients who were classified as being underweight, normal weight, overweight and obese. Comparisons of baseline characteristics between study groups were performed by chi-square and Student’s t test for categorical and continuous variables, respectively.
Univariate and multivariable Cox proportional hazards models were used to assess the associations between obesity and acute rejection, graft loss and patient survival, after adjusting for age at transplant, sex, ethnicity, country, era of transplantation, primary renal disease, comorbidities, the number of HLA mismatches, PRA and donor characteristics (age, sex and whether living or deceased). Collinearity of independent variables was assessed using the variance inflation factor. Variables that had an association with the pre-specified outcomes at a p value of <0.25 in the unadjusted analyses were included in the multivariable analyses. Variables were also considered to be a confounder if they changed the coefficient of the explanatory variable by >10%. Potential effect modification between primary renal disease, age at transplant, year of transplant, HLA mismatch and BMI and outcomes was evaluated, but no significant interactions were found.
The proportional hazard assumptions of all Cox models were assessed by fitting log(time)-dependent covariates in the multivariate models and then plotting the Schoenfeld residuals; all assumptions were found to be met. Results are expressed as hazard ratios (HR) with 95% confidence intervals (95% CI).
Patients were followed from transplant date until death, graft loss or acute rejection. Those who did not experience death, graft loss or acute rejection were censored at the study end date of 31 December 2013, or at 6 months post transplantation for the outcome of acute rejection. For the outcome of death-censored survival, patients were followed from transplant date until graft loss or censored at death or study end date. The proportions free from events were calculated using the Kaplan–Meier method. Sensitivity analyses excluding recipients with early technical losses were also performed. All statistical analyses were performed using Stata version 11.2 (StataCorp LP, College Station TX).
Results
In total, there were 763 eligible patients. Of these, 13 were excluded due to missing data for height. The remaining 750 patients (628 for the outcome of acute rejection) were followed-up for 6597 person-years over a median follow-up time of 8.4 (interquartile range 3.4–19.7) years for the graft survival and patient survival analyses.
Baseline characteristics
The characteristics of the included patients are shown in Table 1. Overall, 77 (10.3%) patients were classified as underweight, 483 (64.4%) as normal weight, 129 (17.2%) as overweight, and 61 (8.1%) as obese at the time of transplant. A small number (n = 15; 2%) of the obese patients were considered to be morbidly obese. Children with obesity were more likely to be younger and have structural urologic primary renal disease, including congenital anomalies of the kidney and urinary tract (CAKUT). Children who were classified as underweight were more likely to be older, have glomerulonephritis as their primary renal disease and were less likely to receive pre-emptive transplantation. Overall, the prevalence of obesity remained relatively unchanged over time (p = 0.2).
Acute rejection within the first 6 months
A total of 102 (16.2%; 95% CI 13.3–19.1%) of the included 628 recipients experienced their first acute rejection within the first 6 months after transplantation as presented in the Kaplan Meier plot shown in Fig. 1. The follow-up period for this analysis was 260 person-years. Compared to children with normal weight, the adjusted hazard ratios for acute rejection in children classified as underweight, overweight and obese were 1.07 (95% CI 0.54–2.09), 1.42 (0.86–2.34) and 1.83 (0.95–3.51), respectively [Electronic Supplementary Material (ESM) Table 1; p value for trend 0.07; see Fig. 4a].
Graft survival
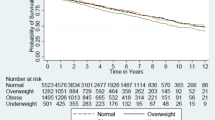
A total of 235 recipients lost their allografts (31.3%; 95% CI 28.0–34.6%), including 21 patients who died with functioning grafts (Fig. 2). The most common causes of graft failure were chronic allograft nephropathy (49%), followed by non-adherence to therapy (11%) and glomerulonephritis (8%). A total of 19 patients experienced early graft loss due to technical factors, such as thrombotic events and surgical complications.
Children with obesity were more likely to experience graft loss than those with normal weight (adjusted HR 1.61, 95% CI 1.05–2.47). Compared to children with normal weight, the adjusted hazard ratios for those children classified as underweight and overweight were 1.05 (95% CI 0.70–1.60) and 1.03 (95% CI 0.71–1.49), respectively (see Fig. 4b and ESM Table 1). No significant interaction terms were found in the models. The findings for death censored graft loss were very similar. Compared to children with normal weight, the adjusted hazard ratios for this outcome among those children classified as underweight, overweight and obese were 0.98 (95% CI 0.63–1.53), 1.16 (95% CI 0.80–1.68) and 1.64 (95% CI 1.05–2.58), respectively. A sensitivity analysis with early graft loss excluded showed no significant change to these results. As shown in Fig. 2, over 10 years, recipients with obesity had a poorer graft survival than those with normal weight. Those children who were underweight or overweight at the time of transplantation appeared to track similarly, between the curves for recipients with normal weight and those with obesity.
Patient survival
Overall 53 children died (7.1%; 95% CI 5.3–8.9%) as presented in the Kaplan Meier plot shown in Fig. 3. The most common causes of death were cardiovascular disease (21%), infection (19%) and malignancy (15%). As seen in Fig. 4c and ESM Table 1, compared with those of normal weight, children who were classified as underweight, overweight and obese did not experience an increased risk of overall death [adjusted HR 1.18 (95% CI 0.54–2.58), 0.85 (95% CI 0.38–1.92) and 0.80 (95% CI 0.25–2.61), respectively].
Adjusted hazard ratios for acute rejection (a), all-cause graft loss (b) and death by BMI categories (c) in pediatric kidney transplant recipients between 1994 and 2013. a Adjusted for age at transplant, country and year of transplant, b adjusted for age at transplant, racial origin, primary renal disease, human leukocyte antigen (HLA) mismatch and year of transplant, c adjusted for age at transplant, HLA mismatch and year of transplant. CI Confidence interval
Discussion
In a population-based study of 750 pediatric kidney transplant recipients with 6597 patient-years of follow-up, we have shown that childhood obesity at transplantation is associated with a 60% increased risk of graft loss. This excess risk manifests soon after transplantation and persists for up to 10 years. Overall, graft loss appears to be the only adverse outcome associated with obesity, with no clear association between obesity and acute rejection or patient survival. Being overweight does not appear to confer a similar risk. Our findings suggest that obesity, affecting 10% of children at transplantation and being a potentially modifiable factor, should be managed effectively before transplantation, and this may prevent unnecessary graft loss.
Our observations may be explained in several ways. One potential contributor to increased graft loss may be attributed to the higher workload at the nephron level in those with obesity, which is manifested in glomerular hyperfiltration and reduced graft function [11]. Glomerular hyperfiltration and hypertrophy as well as hypertension are known non-immunologic contributors to chronic allograft nephropathy, which was the major cause of graft loss in this cohort of pediatric renal transplant recipients. In adults, one of the leading causes of graft and patient loss is cardiovascular risk, in which the role of obesity is well established. Among pediatric renal transplant patients a higher blood pressure has been found in those with obesity post transplant [11, 17], and post-transplant hypertension has been associated with increased chronic allograft injury and poorer graft survival [18]. Given a relatively low event rate for death, this may explain why similar observations have not been found in the relationship between obesity and patient survival. Vascular thrombosis and surgical complications in general are more common in pediatric recipients, and this risk may be enhanced in those with obesity, although such an enhancement was not found in this cohort [19]. Change in BMI over time may also impact upon long-term graft outcomes, but this was beyond the scope of our study. It is also possible that our observation may have simply occurred by chance although this is unlikely as similar results have been reported in the adult population and the effect is biologically plausible.
The association between obesity and recipient outcomes in the adult transplant population has been previously evaluated but with conflicting results. Some of these studies demonstrated that obesity is a risk factor for graft loss, while others found no association between obesity and patient and graft outcomes [20, 21]. A large registry study of over 50,000 adult transplant recipients reported a U-shaped association between BMI and both patient and graft survival. Recipients with a very high and very low BMI at the time of transplant were most at risk for either patient and graft survival [22]. More recently, a number of systematic reviews have assessed the relationship between BMI and graft and patient outcomes post-renal transplantation. The results of these reviews are inconsistent, which in part may be attributed to the different definitions of obesity within BMI that were used. Lafranca et al. reported that obese transplant recipients were more likely to experience acute rejection, wound infection and wound dehiscence than recipients who were not obese [23]. In the same review, the incidence of new-onset diabetes after transplantation and risk of both patient and graft loss at 1, 2 and 3 years after transplantation were also higher in transplant recipients with a BMI of >30 than in those with a BMI of <30 [23]. This differs from studies in which BMI was analyzed using the World Health Organization categories, when both extremes were found to be associated with poorer patient and graft survival outcomes [13].
In the pediatric kidney transplant population, the associations between obesity and graft and patient outcomes are less certain. A study of 6658 pediatric kidney transplant recipients found no reduction in patient and graft survival overall in obese recipients, but a higher risk of death was found in a sub-group of 6- to 12-year-old recipients. This result was attributed largely to cardiovascular disease and vascular thrombosis, although as mentioned this Registry experiences patient loss to follow-up in the adolescent cohort, possibly explaining the lack of similar changes in children older than 12 years [8]. A smaller retrospective study of 76 pediatric patients assessed graft outcomes at 1 year post transplantation. In children who were obese at the time of transplantation there was an increased risk of reduced graft function and increased systolic blood pressure at 1 year; however, there was no difference in acute rejection compared to those who were not obese at the time of transplantation [11].
Our study has a number of strengths. ANZDATA is a comprehensive database of all kidney transplant recipients, which is different from previous registry studies that rely on the voluntary entry of patients. Within ANZDATA, exposure and outcome data are reliably collected and reported from childhood continuing into adulthood [24]. However, although adjustment was made for multiple confounding factors, there may be unmeasured residual confounders, such as type and intensity of maintenance immunosuppression, and remaining co-morbidities that were not reported and so cannot be adjusted for. As records of biopsy-proven rejection and the presence of BK virus-associated nephropathy were not available, misclassification of acute rejection may occur, but this is unlikely to be differential and so would not impact on the associations observed. Given the small numbers of children in each BMI category in our study, we may not have had the statistical power to detect a significant difference in transplant outcomes, including acute rejection and patient survival by BMI groups, and we regard this study as an exploratory one. In addition to this, the very small numbers of morbidly obese children limited assessment of associations of outcomes with BMI in this group or with BMI as a continuous variable. Acute rejection has only been collected through the ANZDATA registry since April 1997, which limits the acute rejection analysis to 84% of the total cohort and reduces power further in this analysis. Having noted an increased risk of graft loss in recipients with obesity, the next steps are to delineate the potential causes and mechanisms of these losses. Further research into the change over time in BMI post transplantation in pediatric recipients may be useful in exploring these long-term associations.
Conclusions
In summary, we have demonstrated an association of obesity and long-term graft outcomes for children with kidney transplants, specifically graft loss. In addition to establishing the causes for these graft losses, concerted efforts to inform parents and children of the possible negative effects of obesity and ensuring children are actively pursuing a healthy lifestyle and dietary choices prior to transplant is a priority.
References
Australian Institute of Health and Welfare (AIHW) (2015) Cardiovascular disease, diabetes and chronic kidney disease—Australian facts: risk factors (full publication; 11 March 2015 edition). AIHW, pp 1–112. Available at: http://aihw.gov.au/publication-detail/?id=60129550538
Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 307:483–490
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NME, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang J-C, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DFJ, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SEAH, Kengne AP, Khader YS, Khang Y-H, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KMV, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJC, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon S-J, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJL, Gakidou E (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013;2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:766–781
Di Bonito P, Sanguigno E, Forziato C, Di Fraia T, Moio N, Cavuto L, Sibilio G, Iardino MR, Di Carluccio C, Capaldo B (2014) Glomerular filtration rate and cardiometabolic risk in an outpatient pediatric population with high prevalence of obesity. Obesity 22:585–589
Nguyen S, McCulloch C, Brakeman P, Portale A, C-y H (2008) Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics 121:37–45
Plumb LA, Pitcher D, Tse Y, Shield JP, Inward C, Sinha MD, Nephrology OBotBAfP (2014) Longitudinal changes in body mass index following renal transplantation in UK children. Nephrol Dial Transplant 29:196–203
Bonthuis M, Van Stralen KJ, Verrina E, Groothoff JW, Alonso Melgar Á, Edefonti A, Fischbach M, Mendes P, Molchanova EA, Paripović D, Peco-Antic A, Printza N, Rees L, Rubik J, Stefanidis CJ, Sinha MD, Zagożdżon I, Jager KJ, Schaefer F (2013) Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. Nephrol Dial Transplant 28 [Suppl 4]: iv195-iv204
Hanevold CD, Ho PL, Talley L, Mitsnefes MM (2005) Obesity and renal transplant outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 115:352–356
Omoloja A, Stolfi A, Mitsnefes M (2005) Pediatric obesity at renal transplantation: a single center experience. Pediatr Transplant 9:770–772
Boschetti SB, Nogueira PCK, Pereira AML, Fisberg M, Pestana JOM (2012) Prevalence, risk factors, and consequences of overweight in children and adolescents who underwent renal transplantation—short- and medium-term analysis. Pediatr Transplant 17:41–47
Mitsnefes MM, Khoury P, McEnery PT (2002) Body mass index and allograft function in pediatric renal transplantation. Pediatr Nephrol 17:535–539
Höcker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, Pohl M, Zimmering M, Fründ S, Klaus G, Wühl E, Tönshoff B (2010) Improved growth and cardiovascular risk after late steroid withdrawal: 2-year results of a prospective, randomised trial in paediatric renal transplantation. Nephrol Dial Transplant 25:617–624
Ahmadi S-F, Zahmatkesh G, Streja E, Molnar MZ, Rhee CM, Kovesdy CP, Gillen DL, Steiner S, Kalantar Zadeh K (2014) Body mass index and mortality in kidney transplant recipients: a systematic review and meta-analysis. Am J Nephrol 40:315–324
Nicoletto BB, Fonseca NKO, Manfro RC, Gonçalves LFS, Leitão CB, Souza GC (2014) Effects of obesity on kidney transplantation outcomes: a systematic review and meta-analysis. Transplantation 98:167–176
Kuczmarski RJ, Ogden CL, Guo SS (2000) CDC growth charts. Vital and Health Statistics of the Centers for Disease Control and Prevention, National Center for Health Statistics, Atlanta. Available at: https://www.cdc.gov/nchs/products/series/series11.htm (No.246)
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Denburg MR, Pradhan M, Shults J, Jones A, Palmer JA, Baluarte HJ, Leonard MB (2010) Longitudinal relations between obesity and hypertension following pediatric renal transplantation. Pediatr Nephrol 25:2129–2139
Sorof JM, Sullivan EK, Tejani A, Portman RJ (1999) Antihypertensive medication and renal allograft failure: a North American Pediatric Renal Transplant Cooperative Study report. J Am Soc Nephrol 10:1324–1330
Irtan S, Maisin A, Baudouin V, Nivoche Y, Azoulay R, Jacqz-Aigrain E, El Ghoneimi A, Aigrain Y (2010) Renal transplantation in children: critical analysis of age related surgical complications. Pediatr Transplant 14:512–519
Gore JL, Pham PT, Danovitch GM, Wilkinson AH, Rosenthal JT, Lipshutz GS, Singer JS (2006) Obesity and outcome following renal transplantation. Am J Transplant 6:357–363
Marcen R, Fernández A, Pascual J, Teruel JL, Villafruela JJ, Rodriguez N, Martins J, Burgos FJ, Ortuno J (2007) High body mass index and posttransplant weight gain are not risk factors for kidney graft and patient outcome. Transplantation 39:2205–2207
Meier-Kriesche H-U, Arndorfer JA, Kaplan B (2002) The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation 73:70–74
Lafranca JA, IJermans JNM, Betjes MGH, Dor FJMF (2015) Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med 13:111
Gray NA, Mahadevan K, Campbell VK, Noble EP, Anstey CM (2013) Data quality of the Australia and New Zealand dialysis and transplant registry: a pilot audit. Nephrology 18:665–670
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare that they have no conflicts of interest.
Funding
ML is the recipient of an NHMRC Postgraduate Scholarships Grant: Clinical Postgraduate Research Scholarship APP1074409.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 82.9 kb)
Rights and permissions
About this article
Cite this article
Ladhani, M., Lade, S., Alexander, S.I. et al. Obesity in pediatric kidney transplant recipients and the risks of acute rejection, graft loss and death. Pediatr Nephrol 32, 1443–1450 (2017). https://doi.org/10.1007/s00467-017-3636-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3636-1