Abstract
Background
Dry weight is the lowest weight patients on hemodialysis can tolerate; correct dry weight estimation is necessary to minimize morbi-mortality, but is difficult to achieve. Here, we used artificial intelligence to improve the accuracy of dry weight assessment in hemodialysis patients.
Methods/Results
We designed a neural network which used bio-impedancemetry, blood volume monitoring, and blood pressure values as inputs; output was artificial intelligence dry weight. Fourteen pediatric patients were switched from nephrologist to artificial intelligence dry weight. Artificial intelligence dry weight was higher (28.6%), lower (50%), or identical to nephrologist dry weight. Mean difference between artificial intelligence and nephrologist dry weights was 0.497 kg (− 1.33 to + 1.29 kg). In patients for whom artificial intelligence dry weight was lower than nephrologist dry weight, systolic blood pressure significantly decreased after dry weight decrease to artificial intelligence dry weight (77th to 60th percentile, p = 0.022); anti-hypertensive treatments were successfully decreased or discontinued in 28.7% of cases. In patients for whom artificial intelligence dry weight was higher than nephrologist dry weight, no hypertension was observed after dry weight increase to artificial intelligence dry weight; when present, symptoms of dry weight underestimation receded.
Conclusions
Neural network predictions outperformed those of experienced nephrologists in most cases, proving artificial intelligence is a powerful tool for predicting dry weight in hemodialysis patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
End-stage renal disease (ESRD) is a public health issue in most developed countries. In the USA, about 70% of ESRD patients are treated with hemodialysis, either temporarily or permanently. ESRD patients on chronic hemodialysis face an increased morbi-mortality compared to kidney transplant recipients [1]; in particular, the prevalence of cardiovascular complications such as hypertension [2] and left ventricular hypertrophy [3] or cardiac ischemia increases as dialysis is maintained. Therefore, to maximize cardiovascular tolerance [4], dialysis parameters need to be optimized; this is particularly true for dry weight (DW) [5].
Dry weight is usually defined as the lowest weight a patient on chronic hemodialysis can tolerate [6]. It has been reported that DW overestimation is responsible for hypertension [7] and cardiac dysfunction, whereas DW underestimation leads to hypotension [8], asthenia, and ischemia. DW management is a complex process; frequent DW updates are often necessary, especially in children, to avoid DW underestimation that occurs owing to patients’ ponderal growth; the same concept is true in malnourished patients, for whom DW overestimation is the main risk.
As a consequence, it is crucial to use an efficient and precise method to determine DW in ESRD patients on chronic hemodialysis.
Historically, DW was determined from clinical examination, looking for edema or cutaneous fold, and from blood pressure measurements [9]. Over the years, paraclinical tools have been developed to help nephrologists assess DW. Measure of the cardio-thoracic ratio on chest X-rays has been used for a long time, but is an imprecise method, as it is dependent on underlying cardiovascular conditions, such as cardiac hypertrophy [10]. Ultrasonic measurement of the inferior vena cava diameter remains controversial, as it seems related to DW in some studies only; moreover, it has not proven to be correlated with DW in pediatric patients [11]. Modern multi-frequency bio-impedancemetry has been validated as part of the strategy to estimate DW [12]; however, due to inter-individual variability, bio-impedancemetry is not usually the only technique used to estimate DW. Thoracic ultrasound could also be a promising tool [10], but is still being evaluated. Finally, blood volume monitoring during hemodialysis has been documented as a valuable help to estimate DW [13]; however, the inter-individual variability of this method remains an issue.
To date, no perfect tool has emerged to precisely assess DW in ESRD patients on chronic hemodialysis; in the absence of clear guidelines, most nephrologists determine DW empirically, using technologies available in their center.
However, such an empirical assessment of DW often proves to be inaccurate [6]. This imprecision jeopardizes dialysis tolerance and increases morbidity, particularly in low-weight infants and children. To improve the accuracy of DW determination, we turned to artificial intelligence [14, 15], a novel and powerful technology.
Patients and methods
Patients
ESRD patients on chronic hemodialysis or hemodiafiltration weighing 20 kg or more were considered between July 1st 2017 and August 2nd 2017 in Robert Debré Hospital.
Neural network
Inputs
Inputs to the neural network were patients’ hydration status measured by bio-impedancemetry (derived from the relative hydration to body weight ratio), relative blood volume measured by blood volume monitoring, and post-dialysis systolic blood pressure.
Output
The output of the neural network was a correction to apply to nephrologist DW to obtain artificial intelligence DW.
Training
Simulated dialysis sessions were created in silico to train the neural network; tuples of simulated inputs were computer-generated along with corresponding simulated output. Neural network training required 11,866 loops and 698 epochs.
Hydration status
Hydration status was measured using bio-impedancemetry (Fresenius Body Composition Monitor), according to the manufacturer’s guidelines, and expressed as a relative percentage of hydration, derived from the relative hydration to body weight ratio.
Relative blood volume
Relative blood volume was monitored during hemodialysis on hemodialysis machines (Fresenius 5008) according to the manufacturer’s recommendations.
Blood pressure
Blood pressure was measured according to the American Heart Association recommendations using oscillatory methods on certified devices, and converted into percentiles based on patients’ age and height. Blood pressures > 90th percentile or < 50th percentile were confirmed by auscultatory methods.
Dialysis
Dialysis sessions
Patients were on hemodialysis or hemodiafiltration depending on their medical history; all patients had 3 dialysis sessions per week; each session lasted 4 h.
Assessment session
Stable patients on chronic hemodialysis were monitored for hydration status, relative blood volume, and post-dialysis blood pressure during assessment sessions. These parameters were used by the neural network to determine artificial intelligence DW.
Follow-up sessions
After validation by expert nephrologists, artificial intelligence DW was used as reference DW for the following dialysis sessions. Tolerance was monitored for at least 3 consecutive follow-up dialysis sessions in terms of inter-dialytic and intra-dialytic undesirable events. Inter-dialytic undesirable events included systolic or diastolic blood pressures above the 75th percentile or significantly below the 50th percentile, asthenia and dizziness; intra-dialytic undesirable events included asthenia, post-dialytic systolic or diastolic blood pressures above the 75th percentile or significantly below the 50th percentile, or clinically relevant blood pressure drops.
Statistics
Statistical analysis was performed using Prism by GraphPad.
Non-parametric Wilcoxon-Mann-Whitney test was used to compare medians.
P values < 0.05 were considered significant.
Results
Network training
We applied artificial intelligence to clinical decision making as we designed, trained, tested, and used a multi-layer perceptron neural network [14] to determine DW in ESRD patients on chronic hemodialysis (Supplementary Fig. 1).
Simulated dialysis sessions were computer-generated to train the neural network: tuples of simulated neural network inputs (relative percentage of hydration, relative blood volume percentage and systolic blood pressure expressed as a percentile) were generated along with their corresponding simulated output (the percentage of correction that should be applied to the simulated DW to obtain an improved DW). Network error after training was 0.00076 (maximum output error tolerance, 0.02).
Network testing and validation
During the test phase, neural network DW predictions were performed in real patients and compared to actual DW estimated by experienced nephrologists based on clinical examination and blood pressure measures, bio-impedancemetry results, cardio-thoracic ratio, and blood volume monitoring analysis. Pearson’s test showed a strong positive correlation between DW predicted by the neural network during the test phase, and observed DW in stable patients (R = 0.9997, p < 0.00001).
Population characteristics
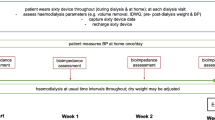
Once validated, the neural network was used to prospectively predict DW in 14 patients on chronic hemodialysis who were switched from nephrologist dry weight (N-DW) to artificial intelligence dry weight (AI-DW) as shown in Fig. 1. Sex ratio was 71% male, mean age was 13.7 years (7 to 17 years), and mean weight was 40.9 kg (20 to 60.2 kg).
Flowchart of the study. Parameters required by the neural network to determine artificial intelligence dry weight (AI-DW) were obtained in stable patients on chronic hemodialysis during assessment session. Patients were then switched from nephrologist dry weight (N-DW) to AI-DW and monitored for at least 3 consecutive follow-up hemodialysis sessions
Network predictions
Artificial intelligence dry weight was higher (28.6% of cases), lower (50%), or identical (21.4%) to N-DW. Mean difference between N-DW and AI-DW was 0.497 kg (− 1.33 to + 1.29 kg). There was no significant difference between AI-DW and DW that could be achieved in practice after the patients were switched to AI-DW (p = 0.99).
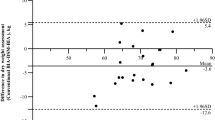
A Bland-Altman plot of AI-DW and N-DW showed a bias of − 0.098; 95% limits of agreement ranged from − 1.36 to 1.17, as shown in Supplementary Fig. 2.
Evaluation of DW control
In patients for whom AI-DW was lower than N-DW, AI-DW was evaluated in terms of blood pressure control, limitation of anti-hypertensive treatments, and clinical tolerance. Median systolic blood pressure significantly decreased after DW adjustment to AI-DW (77th percentile to 60th percentile, p = 0.022). Anti-hypertensive treatments were successfully decreased or discontinued in 28.7% of cases. Direct consequences of blood pressure decrease on left ventricular mass were not evaluated in this study; however, it has been shown that better blood pressure control improves cardiovascular outcome in patients on chronic hemodialysis [2]. No intra-dialytic or inter-dialytic undesirable events were reported in these patients.
In patients for whom AI-DW was higher than N-DW, AI-DW was evaluated in terms of blood pressure control, absence of initiation of anti-hypertensive treatments, and clinical tolerance. No significant change in median systolic blood pressure occurred after DW adjustment to AI-DW (67th to 68th percentile, p = 0.99). Before increasing DW from N-DW to AI-DW, one patient presented intra-dialytic blood pressure drop, and two patients complained of mild inter-dialytic asthenia; after increasing DW to AI-DW, no intra-dialytic or inter-dialytic undesirable events were reported. No anti-hypertensive treatment had to be initiated in these patients.
Notably, no significant difference in terms of tolerance or blood pressure values was noticed between patients on hemodialysis or hemodiafiltration.
Discussion
Artificial intelligence in general—and neural networks in particular—is a powerful approach to approximate functions which depend on several variables in a complex, non-linear way [14]. Here, we hypothesized that DW was in the co-domain of a function which could be successfully approximated using 3 main variables: patients’ hydration status, relative blood volume, and blood pressure; these variables have all been reported to be correlated to DW [9, 12, 13]. In our study, neural network DW predictions outperformed those of experienced nephrologists in most cases. Interestingly, variables required by the neural network to determine AI-DW are validated, cheap, and easy to obtain, as most centers already use bio-impedancemetry, blood volume monitoring, and blood pressure measures to appreciate dialysis tolerance. Notably, collecting these data is a non-invasive process, which is an important argument when taking care of children.
From a technical point of view, neural network interface was user-friendly and could be accessed online through the Internet, on a computer or a smartphone; this ease of use is an important criterion for artificial intelligence deployment.
The main downside of artificial intelligence is the necessity to retrain the neural network whenever a significant change is made in the target population, or in the way an input is measured; for example, changing relative blood volume or hydration status measuring techniques would require a retraining of the network. Moreover, managing an artificial intelligence algorithm requires specific competences that are beyond the scope of pure medical training; it necessitates a multidisciplinary approach, involving computer scientists, mathematicians, and physicians.
In conclusion, artificial intelligence improved DW estimation and hemodialysis tolerance in pediatric patients. Further studies are required to assess the impact of the better DW control obtained with artificial intelligence, in particular in terms of cardiovascular complications.
References
Neu AM, Frankenfield DL (2009) Clinical outcomes in pediatric hemodialysis patients in the USA: lessons from CMS’ ESRD CPM project. Pediatr Nephrol 24:1287–1295. https://doi.org/10.1007/s00467-008-0831-0
Chavers BM, Solid CA, Daniels FX, Chen SC, Collins AJ, Frankenfield DL, Herzog CA (2009) Hypertension in pediatric long-term hemodialysis patients in the United States. Clin J Am Soc Nephrol 4:1363–1369. https://doi.org/10.2215/CJN.01440209
Peng W, Li Z, Xu H, Xia C, Guo Y, Zhang J, Liu K, Li Y, Pu J, Zhang H, Cui T (2018) Assessment of right ventricular dysfunction in end-stage renal disease patients on maintenance haemodialysis by cardiac magnetic resonance imaging. Eur J Radiol 102:89–94. https://doi.org/10.1016/j.ejrad.2018.02.036
Fischbach M, Zaloszyc A, Shroff R (2015) The interdialytic weight gain: a simple marker of left ventricular hypertrophy in children on chronic haemodialysis. Pediatr Nephrol 30:859–863. https://doi.org/10.1007/s00467-015-3086-6
Gotta V, Marsenic O, Pfister M (2018) Age- and weight-based differences in haemodialysis prescription and delivery in children, adolescents and young adults. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfy067
Raimann J, Liu L, Tyagi S, Levin NW, Kotanko P (2008) A fresh look at dry weight. Hemodial Int 12:395–405. https://doi.org/10.1111/j.1542-4758.2008.00302.x
Heerspink HJL, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V (2009) Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet 373:1009–1015. https://doi.org/10.1016/S0140-6736(09)60212-9
Sands JJ, Usvyat LA, Sullivan T, Segal JH, Zabetakis P, Kotanko P, Maddux FW, Diaz-Buxo JA (2014) Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int 18:415–422. https://doi.org/10.1111/hdi.12138
Paglialonga F, Consolo S, Edefonti A, Montini G (2018) Blood pressure management in children on dialysis. Pediatr Nephrol 33:239–250. https://doi.org/10.1007/s00467-017-3666-8
Allinovi M, Saleem MA, Burgess O, Armstrong C, Hayes W (2016) Finding covert fluid: methods for detecting volume overload in children on dialysis. Pediatr Nephrol 31:2327–2335. https://doi.org/10.1007/s00467-016-3431-4
Torterüe X, Dehoux L, Macher MA, Niel O, Kwon T, Deschênes G, Hogan J (2017) Fluid status evaluation by inferior vena cava diameter and bioimpedance spectroscopy in pediatric chronic hemodialysis. BMC Nephrol 18:373. https://doi.org/10.1186/s12882-017-0793-1
Wong Vega M, Srivaths PR (2017) Air displacement plethysmography versus bioelectrical impedance to determine body composition in pediatric hemodialysis patients. J Ren Nutr 27:439–444. https://doi.org/10.1053/j.jrn.2017.04.007
Srisuwan K, Hongsawong N, Lumpaopong A, Thirakhupt P, Chulamokha Y (2015) Blood volume monitoring to assess dry weight in pediatric chronic hemodialysis patients. J Med Assoc Thail 98:1089–1096
Nielsen MA (2015) Neural Networks. Neural Networks and Deep Learning, Determination Press
Gil Y, Greaves M, Hendler J, Hirsh H (2014) Artificial intelligence. Amplify scientific discovery with artificial intelligence. Science 346:171–172. https://doi.org/10.1126/science.1259439
Acknowledgments
The authors would like to thank the nurses at Robert Debré Pediatric Nephrology Department for their help in assessing dialysis parameters during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Niel, O., Bastard, P., Boussard, C. et al. Artificial intelligence outperforms experienced nephrologists to assess dry weight in pediatric patients on chronic hemodialysis. Pediatr Nephrol 33, 1799–1803 (2018). https://doi.org/10.1007/s00467-018-4015-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4015-2