Abstract
Background
Traditional serological biomarkers often fail to assess systemic lupus erythematosus (SLE) disease activity and discriminate lupus nephritis (LN). The aim of this study was to identify novel markers for evaluating renal and overall disease activity in Chinese patients with pediatric systemic lupus erythematosus (pSLE).
Methods
The study included 46 patients with pSLE (35 girls, 11 boys; average age 13.3 ± 2.6 years) and 31 matched healthy controls (22 girls, 9 boys; average age 12.3 ± 2.4 years). The SLE Disease Activity Index (SLEDAI) and renal SLEDAI were used to assess disease activity. Nine different soluble mediators in plasma, including tumor necrosis factor alpha (TNF-α), platelet-derived growth factor-BB (PDGF-BB), interferon (IFN) gamma inducible protein 10 (IP-10), interleukin (IL)-1β, IFN-γ, IL-17A, IL-2, Fas and Fas ligand, were measured by Luminex assay and compared between patients with active and inactive pSLE as well as between patients with pSLE with active and inactive renal disease. Receiver operating characteristic curve analysis was used to measure the discrimination accuracy.
Results
Of the 46 patients with pSLE, 30 (65.2%) had LN. These patients had significantly elevated levels of serum TNF-α, PDGF-BB, IP-10 and Fas. The serum levels of IP-10 were also significantly higher in patients with active pSLE. We found that IP-10 was also more sensitive and specific than conventional laboratory parameters, including anti-double-stranded DNA and complement components C3 and C4, for distinguishing active lupus from quiescent lupus. The serum level of IP-10 was also significantly increased in children with pSLE with active renal disease relative to those with inactive renal disease. There was also a positive correlation between serum IP-10 levels and renal SLEDAI scores as well as with 24 h urine protein.
Conclusions
Serum IP-10 is useful for identifying renal and overall disease activity in children with pSLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease, characterized by multi-systemic involvement, with a wide range of clinical presentations and laboratory manifestations [1]. Lupus nephritis is one of the most common and devastating complications of SLE, with the resulting renal damage more prevalent and aggressive in pediatric patients than in adult ones [2,3,4,5,6,7]. Diverse serological and clinical manifestations are observed among SLE patients, and these represent a real challenge to clinicians evaluating disease activity and adjusting medication therapy [8, 9]. Traditional serological biomarkers of disease activity, including anti-double-stranded DNA (anti-dsDNA) antibodies and complement components, have been demonstrated to be unreliable parameters of disease activity [10, 11]. The lack of useful biomarkers hampers the evaluation of disease activity and hence impedes appropriate treatment.
Chronic inflammation is closely related with the progression and relapse of SLE. Cytokines and chemokines not only have the biological function of promoting immune cell maturation, they also have the capacity to induce, maintain and amplify inflammatory responses [12,13,14,15]. A series of markers to reflect disease activity in SLE have been identified in previous studies, including serum interferon alpha (IFN-α) and interleukin 6 (IL-6) [16, 17]. Recent studies have also demonstrated that serum colony-stimulating factor-1, urinary monocyte chemoattractant protein-1, transferrin, IL-27 and IL-23 are excellent indicators of renal disease activity in SLE [18,19,20,21,22]. However, only a few studies have investigated the cytokine profile of pediatric SLE (pSLE) systemically. Therefore, it is of great significance to explore novel biomarkers of disease activity that are also specific for lupus nephritis in pSLE.
IFN-γ inducible protein-10 is also known as IP-10 (CXCL10) and its receptor CXCR3 are expressed by activated T helper (Th) cells, B cells, macrophages and natural killer (NK) cells [23]. Therefore IP-10 may attract these cells to inflammatory sites where they exert a wide-ranging impact; as such, serum IP-10 may be a pathogenic factor and a potential biomarker in SLE patients. In addition, previous studies have demonstrated that platelet-derived growth factor-B (PDGF-B) has a high affinity for PDGFR-β expressed on cultured human mesangial cells, contributing to both their proliferation and extracellular matrix accumulation [24], which is the main feature of lupus nephritis (LN). Thus, PDGF-BB is another inflammatory cytokine that has drawn our attention in the study. Last but not the least, apoptosis-related molecules, such as Fas and Fas ligand (FasL), also play a vital role in the regulation of immune tolerance and may be involved in the pathogenesis of SLE. To date, no researchers have investigated the circulating levels of these related proteins systemically and determined whether some of them could reflect disease activity or renal status in Chinese pSLE.
Therefore, the aim of our study was to investigate the levels of the nine different soluble mediators mentioned above [including Th1 cytokines, such as IFN-γ, IL-2, tumor necrosis factor alpha (TNF-α), the Th17-related cytokine IL-17A, IL-1β as well as IP-10, PDGF-BB and the apoptosis-related proteins Fas and FasL] in pSLE to analyze their association with clinical disease activity and renal status. We also evaluated the potential value of these mediators in identifying active pSLE as well as patients with active renal disease.
Subjects and methods
Subjects
Forty-six patients diagnosed with SLE, with age at onset of < 18 years according to the Pediatric Rheumatology International Trials Organization (PRINTO), and 31 gender- and age-matched healthy controls (HCs) were included in this study. All patients fulfilled at least four of the American College of Rheumatology classification criteria for SLE [25]. This study was approved by the Ethics Committee of Shanghai Children’s Medical Center, Shanghai, China, and written informed consent was obtained from each participant in accordance with institutional guidelines.
Clinical data and sample collection
The demographic, clinical and laboratory features of all patients were collected in our medical database. The laboratory measurements included white blood cell counts, lymphocyte, platelet counts, hemoglobin, C-reactive protein, erythrocyte sedimentation rate (ESR), serum creatinine, serum albumin, 24-h urinary protein excretion, routine urine sample analysis, serum C3 and C4 levels and autoantibodies such as antinuclear antibodies and anti-dsDNA levels, among others. Clinical disease activity was assessed using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). Blood was collected from the 46 patients with pSLE and 31 gender- and age-matched HCs. Plasma was isolated and stored at − 80 °C until analysis.
Detection of serum protein markers
The serum levels of TNF-α, PDGF-BB, IP-10, IL-1β, IFN-γ, IL-17A, IL-2, Fas and FasL were determined using the Human Premixed Multi-Analyte kit (Magnetic Luminex Screening Assay; R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Assessment of clinical disease activity and flares
Clinical disease activity was evaluated using the SLEDAI. SLE flares were evaluated using the SELENA (Safety of Estrogens in Lupus Erythematosus National Assessment) flare index as described previously by Buyon et al. [26] on the basis of the clinical status of the pSLE upon blood draw. Clinically active SLE was defined as a SLEDAI score of > 4 or the presence of SLE flares; clinically inactive SLE was categorized as a SLEDAI score of ≤ 4. Lupus nephritis was diagnosed according to the following criteria: persistent 24-h proteinuria of > 500 mg or > 3+ by dipstick or cellular casts, including red blood cells, hemoglobin, granular, tubular or mixed [27]. Types of renal nephritis were evaluated according to the 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) lupus nephritis classification system [28]. The cohort was also classified into those with pSLE with active renal disease and those with pSLE with inactive renal disease according to the renal SLEDAI (rSLEDAI) scores. The rSLEDAI score was evaluated based on the four components of the urine analysis, including proteinuria, urinary casts, hematuria and leucocyturia in SLEDAI. SLE was classified as active renal disease if the rSLEDAI score was ≥ 4 and as inactive renal disease if the rSLEDAI score was 0 [29].
Statistical analyses
All data were expressed as mean ± standard deviation (SD). Levels of the markers were compared using the nonparametric Mann–Whitney U test (between two groups) and the Kruskal–Wallis H test (among three groups). The nonparametric Spearsman’s rank correlation test was used to evaluate the correlation between the concentration of IP-10 and the SLEDAI and rSLEDAI scores.
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each of the markers for the assessment of SLE disease activity were calculated. A positive outcome referred to active disease activity (SLEDAI > 4). Elevated anti-dsDNA was defined as titers above the cutoff value for positivity according to the test kits. Low C3 and C4 levels were defined as levels below the normal minimum cutoff value (90 mg/dl for C3 and 10 mg/dl for C4) according to the test kits. For the marker IP-10, an increase was defined as a concentration above the cutoff value at the optimal sensitivity and specificity in the receiver operating characteristic (ROC) curve. Sensitivity was a true positive (TP) divided by the sum of the TP and false negative (FN). Specificity was the radio of true negative (TN) to the sum of TN and false positive (FP). The PPV was the ratio of TP to the sum of TP and FP, and the NPV was the ratio of TN to the sum of TN and FN. To evaluate the diagnostic performance, sensitivity was plotted against (1 − specificity) at different cutoff values for protein levels by ROC analysis. A P value of < 0.05 was considered to be statistically significant. All statistical analyses were carried out using Statistical Package for the Social Sciences, version 22.0 (IBM Corp., Armonk, NY) and GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA).
Results
Demographics and the baseline characteristics
A total of 46 patients with pSLE (35 girls, 11 boys; average age 13.3 ± 2.6 years) and 31 age- and gender-matched HCs (22 girls, 9 boys;; average age 12.3 ± 2.4 years) were included in the study. Clinically active pSLE was present in 27 of the 46 patients (58.7%) and active renal disease was present in 18 of these patients (39.1%) at the time of blood draw. The mean (± SD) age at diagnosis and average disease duration were 10.6 ± 2.8 and 2.7 ± 1.8 years, respectively. The mean score of SLEDAI upon blood draw was 4.35 ± 3.35. Of the 46 patients with pSLE, 30 (65.2%) were diagnosed with LN; of these latter 30 patients, kidney biopsy was performed in 20, with the resulting histology being class IV in ten biopsies, class V in four biopsies, mixed type in three biopsies, class II in two biopsies and class III in one biopsy (Table 1).
Elevated serum levels of TNF-α, PDGF-BB, IP-10 and Fas in patients with pSLE
Nine different soluble proteins, including TNF-α, PDGF-BB, IP-10, IL-1β, IFN-γ, IL-17A, IL-2, Fas and FasL, were analyzed in the plasma obtained from the 46 patients with pSLE and 31 gender- and age-matched HCs. There was a significant difference in the mean levels of TNF-α (9.53 ± 10.91 vs. 2.45 ± 1.56 pg/ml; p = 0.0006), PDGF-BB (351.31 ± 125.50 vs. 169.32±113.04 pg/ml; p < 0.0001), IP-10 (23.62 ± 23.35 vs. 2.90 ± 1.15 pg/ml; p < 0.0001) and Fas (595.43 ± 313.82 vs. 413.92 ± 121.41 pg/ml; p = 0.0029) between patients with pSLE and the HCs, respectively (Fig. 1). Plasma IL-1β (3.90 ± 4.90 vs. 2.26 ± 1.29 pg/ml; p = 0.0724), IFN-γ (48.23 ± 67.22 vs. 59.71 ± 74.94 pg/ml; p = 0.4788), IL-17A (3.32 ± 1.52 vs. 3.52 ± 2.18 pg/ml; p = 0.6351), IL-2 (27.76 ± 24.60 vs. 26.11 ± 15.84 pg/ml; p = 0.7403) and FasL (7.51 ± 3.76 vs. 7.77 ± 2.96 pg/ml; p = 0.7431) did not differ significantly between the pSLE and HC groups, respectively (data not shown).
Serum concentrations of tumor necrosis factor alpha (TNF-α), platelet-derived growth factor-BB (PDGF-BB), interferon (IF) gamma inducible protein 10 (IP-10) and Fas in patients with pediatric systemic lupus erythematosus (pSLE) and age- and gender-matched healthy controls (pHC). Patients with pSLE had a significantly increased concentration of serum TNF-α (***significant difference at p < 0.001), PDGF-BB (****significant difference at p < 0.0001), IP-10 (****significant difference at p < 0.0001) and Fas (**significant difference at p < 0.01) compared with their matched HCs
Elevated serum levels of IP-10 in active pSLE
We also compared the levels of TNF-α, PDGF-BB, IP-10 and Fas in patients with active or inactive pSLE. We found that the serum levels of IP-10 were significantly increased in patients with active pSLE compared with the HCs and patients with inactive pSLE (32.98 ± 27.81 vs. 2.90 ± 1.15 vs. 12.92 ± 6.15 pg/ml, respectively). The results are shown in Fig. 2a.
Serum IP-10 for identifying clinical disease activity in patients with pSLE. a Serum concentrations of IP-10 in patients with active pSLE or inactive pSLE and in healthy controls (HC). Active SLE vs. HC, ****significant difference at p < 0.0001; active SLE vs. inactive SLE, **significant difference at p < 0.01; inactive SLE vs. HC, ****significant difference at p < 0.0001). b Concentrations of serum IP-10 correlated with the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (p < 0.0001, R 2 = 0.3335). c Receiver-operating characteristic (ROC) curves of serum IP-10 for the identification of active pSLE. AUC Area under the concentration–time curve, CI confidence interval
Serum IP-10 levels discriminate between active and inactive disease
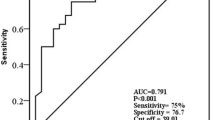
The nonparametric Spearman’s rank correlation test was performed to evaluate the correlation between serum concentrations of IP-10 and SLEDAI. As shown in Fig. 2b, there was an obvious positive correlation between serum IP-10 concentration and SLE disease severity as measured by SLEDAI. ROC curve analysis was carried out to establish the optimal discriminatory threshold to discriminate patients with pSLE with active disease from those with inactive disease, based on serum IP-10 levels (Fig. 2c). At the optimal cutoff point of 14.41 pg/ml of IP-10, the area under the curve for IP-10 serum levels that differentiated active pSLE from inactive pSLE was 0.807 [95% confidence interval (CI) 0.680–0.934, p < 0.0001] with a sensitivity of 0.815 and specificity of 0.737.
Sensitivity and specificity of serum IP-10 for discriminating disease activity
The sensitivity, specificity and PPV/NPV of IP-10 for ascertaining clinically active pSLE are shown in Table 2. Serum IP-10 was more sensitive and specific than conventional markers (elevated anti-dsDNA, decreased C3 and C4 levels) in identifying patients with active disease. The PPV and NPV of elevated IP-10 for discriminating active pSLE were higher than those of elevated anti-dsDNA and low C3 and C4 values.
Serum IP-10 levels for discriminating pSLE with active renal disease
Since LN is a feature of pSLE, we continued to investigate the level of serum IP-10 in patients with active renal disease and those with inactive renal disease. We found that patients with active renal disease had a significantly increased serum level of IP-10 than the HCs and those with inactive renal disease (36.54 ± 32.70 vs. 2.90 ± 1.15 vs. 17.09 ± 10.46 pg/ml, respectively; p < 0.05) (Fig. 3a). In addition, the two groups (active disease vs. HCs/inactive disease) could be discriminated based on serum IP-10 levels. The ROC curve analysis to discriminate patients with active renal disease based on serum IP-10 level is shown in Fig. 3b. At the optimal cutoff point of 16.27 pg/ml of IP-10, the area under the curve for serum IP-10 level that differentiated patients with pSLE with active renal disease from those with pSLE with inactive renal disease was 0.712 (95% CI 0.545–0.879, p = 0.016) with a sensitivity of 0.722 and specificity of 0.607.
Serum IP-10 levels for discriminating patients with pSLE with active renal disease. a Serum concentrations of IP-10 in patients with pSLE with active renal disease and in those with inactive renal disease. Active renal disease vs. HC, ****significant difference at p < 0.0001; active renal disease vs. inactive renal disease, **significant difference at p < 0.01; inactive renal disease vs. HC, ****significant difference at p < 0.0001. b ROC curves of serum IP-10 for the identification of patients with pSLE with active renal disease
Serum IP-10 level correlates with renal SLEDAI score and 24 h urine protein
Since serum IP-10 could discriminate active renal disease, we further to investigate the correlation between the serum concentration of IP-10 and renal SLEDAI score as well as 24 h urine protein. The correlation analysis showed a significant positive correlation between the serum concentration of IP-10 and renal SLEDAI score (p < 0.0001, R 2 = 0.3575) as well as 24 h urine protein (p = 0.0122, R 2 = 0.1343) in pSLE (Fig. 4).
Positive correlation between concentrations of serum IP-10 and scores of renal SLEDAI as well as 24 h urine protein. a There was a positive correlation (p < 0.0001, R 2 = 0.3575) between concentrations of serum IP-10 and score of renal SLEDAI (rSLEDAI) in patients with pSLE. b There was a positive correlation (p = 0.0122, R 2 = 0.1343) between concentrations of serum IP-10 and 24 h urine protein in patients with pSLE
Discussion
Chronic inflammation is closely related with the progression and relapse of SLE. It has been reported that cytokines play vital roles in the modulation of systemic inflammation and local tissue damage [12,13,14,15]. In the present study, we have demonstrated elevated serums level of TNF-α, PDGF-BB, IP-10 and Fas in Chinese patients with pSLE. In addition, we have also shown that serum IP-10 was not only a more sensitive and specific biomarker than conventional parameters in distinguishing disease activity but that it can also discriminate active renal disease from inactive renal disease in our cohort of pSLE. Particularly, we observed a positive correlation between serum IP-10 concentrations and renal SLEDAI as well as 24 h urine protein. This is the first study to have systemically evaluated a series of cytokines and to have proved that serum IP-10 is useful in identifying renal and overall disease activity in Chinese patients with pSLE.
A number of studies have suggested that a TNF-α gene polymorphism is involved in the susceptibility to SLE [30,31,32]. Our results showed a significantly increased level of serum TNF-α in the patients with pSLE, which was also reported in previous studies [33, 34]. As an important inflammatory cytokine, TNF-α may promote a derangement in immune regulation and be a key factor in the pathogenesis of pSLE. Although we found no distinct difference in the serum level of TNF-α between patients with active pSLE and those with quiescent SLE, it is likely that TNF-α concentration is reflective of the inflammatory status of the pSLE patients because a majority of those with pSLE with fever and a concurrent increased level of ESR also demonstrated a high serum TNF-α level at the same time (data not shown).
The PDGF family is composed of PDGF-A, PDGF-B, PDGF-C and PDGF-D. The original members of the PDGF family are processed intracellularly and secreted in forms of disulfide-bonded homodimers or heterodimers (PDGF-AA, PDGF-BB and PDGF-AB). It is recognized that PDGF-B signaling through PDGFR-β plays a pivotal role in glomerular mesangial cell proliferation and interstitial fibrosis [35, 36]. In animal models, infusing or transfecting mice with PDGF-BB contributes to mesangial cell proliferation and matrix accumulation in kidneys [37, 38]. Taken together these findings suggest a possible role of PDGF-BB in renal disease in which glomerular mesangial cell proliferation and interstitial fibrosis account for the majority of manifestations. Nevertheless, there have been, to our knowledge, no reports on the plasma level of PDGF-BB in patients with pSLE to date. Based on the results of our study, we report, for the first time, significantly elevated plasma levels of PDGF-BB in patients with pSLE compared with the HCs. It is noteworthy that the levels of serum PDGF-BB in patients with pSLE who had ever suffered from LN were increased compared to those with pSLE who had never experienced LN—even though the level of serum PDGF-BB in those patients with pSLE with active renal disease was similar to that in patients with pSLE with inactive renal disease at blood draw (data not shown). This result indicates that the level of serum PDGF-BB can not be used as a real time measurement for detecting renal status.
Apoptosis-related molecules Fas can be found in serum as a soluble form. In contrary to the membrane-bound Fas molecule, serum Fas may block the function of FasL on the cell surface and inhibit CD95–CD95L interaction, thereby protecting against apoptosis [39, 40]. Therefore, an increased level of serum Fas may give rise to autoimmunity and account for the breakage of tolerance in pSLE.
IP-10 is secreted by a cluster of cells, including T lymphocytes, NK cells, monocytes and endothelial cells upon stimulation by IFN gamma. It interacts with its receptor CXCR3 on T cells and is involved in the mediation of their trafficking into the inflammatory lesions [41]. Our study showed that the serum concentration of IP-10 was significantly elevated in patients with pSLE compared to the HCs as well as in those with active pSLE compared to patients with quiescent pSLE. Although these results are similar to those previously reported [42,43,44], to the best of our knowledge, our study is the first to demonstrate elevated levels of IP-10 in Chinese patients with pSLE. We have also demonstrated that serum IP-10 was more sensitive and specific than conventional measurements, such as elevated anti-dsDNA, low C3 and C4 levels, in differentiating active pSLE. The role of IP-10 in LN was not consistent in previous studies, with some demonstrating that IP-10 was not indicative of renal activity [43, 45] and others demonstrating the opposite [46, 47]. This may be due to differences in ethnic backgrounds, renal disease severity and detection methods. Our results indicate that serum IP-10 was able to discriminate patients with pSLE with active renal disease although this capacity was limited (area under the time–concentration curve = 0.712), possibly due to the small sample size in the study. The significance of elevated levels of serum IP-10 in patients with pSLE with active renal disease is supported by the results of a previous study in which IP-10 receptor CXCR3 was detected in renal tubulointerstitial infiltrates [48]. Thus, we postulate that an increased serum level of IP-10 can be oriented towards the renal infiltrates and exert an inflammatory effect in kidney in patients with pSLE with active renal disease. In support of this hypothesis, another study has not only shown a positive correlation between the receptor of IP-10, CXCR3 expression in glomerular and proteinuria, but also demonstrated that the receptor of IP-10, CXCR3, in tubulointerstitial significantly correlated with serum creatinine [49]. Thus, an increased serum level of IP-10 may participate in the pathogenesis of LN and have the potential to be a biomarker for identifying active renal disease in lupus.
Undoubtedly, cytokines and chemokines secreted locally within the kidney are instrumental in the pathogenesis of LN. Their excretion in the urine is an excellent indicator of their local production and may better reflect the inflammatory microenvironment in the kidney than serum-based markers. However, according to previous publications, the concentration of urinary IP-10 was much lower than that of serum IP-10 [45]. Thus, the measurement of urinary IP-10 levels in clinical practice requires a more sensitive detection ability and advanced equipment.
We recognize the potential limitations of our study. Since the results were from a single-center study with a relatively small sample size, they need to be confirmed in a larger cohort. Also, due to the limitation of time, we were not able to obtain plasma samples from the same individual over time and monitor the levels of these proteins dynamically. However, here we present the initial evidence that there was an imbalanced cytokine profile in our patients with pSLE, and we have demonstrated that serum IP-10 was associated with clinical disease activity in our patient cohort, indicating that IP-10 can be an indication for monitoring renal disease activity in patients with pSLE.
Conclusions
Our study demonstrated that serum IP-10 can be used as a novel biomarker for identifying disease activity in patients with pSLE. In addition, serum IP-10 is a potential candidate specific for lupus renal disease which should be further explored in a larger cohort of patients with pSLE.
References
Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365:2110–2121
Klein-Gitelman M, Reiff A, Silverman ED (2002) Systemic lupus erythematosus in childhood. Rheum Dis Clin North Am 28:561–577
Stichweh D, Arce E, Pascual V (2004) Update on pediatric systemic lupus erythematosus. Curr Opin Rheumatol 16:577–587
Brunner HI, Gladman DD, Ibanez D, Urowitz MD, Silverman ED (2008) Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 58:556–562
Mina R, Brunner HI (2010) Pediatric lupus--are there differences in presentation, genetics, response to therapy, and damage accrual compared with adult lupus? Rheum Dis Clin North Am 36:53–80
Tarr T, Derfalvi B, Gyori N, Szanto A, Siminszky Z, Malik A, Szabo AJ, Szegedi G, Zeher M (2015) Similarities and differences between pediatric and adult patients with systemic lupus erythematosus. Lupus 24:796–803
Hoffman IE, Lauwerys BR, De Keyser F, Huizinga TW, Isenberg D, Cebecauer L, Dehoorne J, Joos R, Hendrickx G, Houssiau F, Elewaut D (2009) Juvenile-onset systemic lupus erythematosus: different clinical and serological pattern than adult-onset systemic lupus erythematosus. Ann Rheum Dis 68:412–415
Huggins JL, Holland MJ, Brunner HI (2016) Organ involvement other than lupus nephritis in childhood-onset systemic lupus erythematosus. Lupus 25:857–863
Weiss JE, Sison CP, Ilowite NT, Gottlieb BS, Eberhard BA (2007) Flares in pediatric systemic lupus erythematosus. J Rheumatol 34:1341–1344
Liu CC, Manzi S, Ahearn JM (2005) Biomarkers for systemic lupus erythematosus: a review and perspective. Curr Opin Rheumatol 17:543–549
Esdaile JM, Abrahamowicz M, Joseph L, MacKenzie T, Li Y, Danoff D (1996) Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail. Arthritis Rheum 39:370–378
Jacob N, Stohl W (2011) Cytokine disturbances in systemic lupus erythematosus. Arthritis Res Ther 13:228
Munroe ME, Vista ES, Guthridge JM, Thompson LF, Merrill JT, James JA (2014) Proinflammatory adaptive cytokine and shed tumor necrosis factor receptor levels are elevated preceding systemic lupus erythematosus disease flare. Arthritis Rheumatol 66:1888–1899
Gröndal G, Gunnarsson I, Rönnelid J, Rogberg S, Klareskog L, Lundberg I (2000) Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 18:565–570
Adhya Z, Borozdenkova S, Karim MY (2011) The role of cytokines as biomarkers in systemic lupus erythematosus and lupus nephritis. Nephrol Dial Transplant 26:3273–3280
Becker-Merok A, Ostli-Eilersten G, Lester S, Nossent J (2013) Circulating interferon-α2 levels are increased in the majority of patients with systemic lupus erythematosus and are associated with disease activity and multiple cytokine activation. Lupus 22:155–163
Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM, Kim SH, Park HS, Suh CH (2007) Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol 27:461–466
Menke J, Amann K, Cavagna L, Blettner M, Weinmann A, Schwarting A, Kelley VR (2015) Colony-stimulating factor-1: a potential biomarker for lupus nephritis. J Am Soc Nephrol 26:379–389
Smith EM, Jorgensen AL, Midgley A, Oni L, Goilav B, Putterman C, Wahezi D, Rubinstein T, Ekdawy D, Corkhill R, Jones CA, Marks SD, Newland P, Pilkington C, Tullus K, Beresford MW (2017) International validation of a urinary biomarker panel for identification of active lupus nephritis in children. Pediatr Nephrol 32(2):283–295
Watson L, Tullus K, Pilkington C, Chesters C, Marks SD, Newland P, Jones CA, Beresford MW (2014) Urine biomarkers for monitoring juvenile lupus nephritis: a prospective longitudinal study. Pediatr Nephrol 29(3):397–405
Marks SD, Shah V, Pilkington C, Tullus K (2010) Urinary monocyte chemoattractant protein-1 correlates with disease activity in lupus nephritis. Pediatr Nephrol 25(11):2283–2288
Xia LP, Li BF, Shen H, Lu J (2015) Interleukin-27 and interleukin-23 in patients with systemic lupus erythematosus: possible role in lupus nephritis. Scand J Rheumatol 44:1–6
Neville LF, Mathiak G, Bagasra O (1997) The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev 8:207–219
Johnson RJ, Floege J, Couser WG, Alpers CE (1993) Role of platelet-derived growth factor in glomerular disease. J Am Soc Nephrol 4:119–128
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, Merrill JT, Sammaritano L, Lockshin M, Alarcón GS, Manzi S, Belmont HM, Askanase AD, Sigler L, Dooley MA, Von Feldt J, McCune WJ, Friedman A, Wachs J, Cronin M, Hearth-Holmes M, Tan M, Licciardi F (2005) The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med 142:953–962
Hahn BH, MA MM, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi SI, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM, American College of Rheumatology (2012) American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64:797–808
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M, International Society of Nephrology Working Group on the Classification of Lupus Nephritis (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65:521–530
Gupta R, Aggarwal A, Sinha S, Rajasekhar L, Yadav A, Gaur P, Misra R, Negi VS (2016) Urinary osteoprotegerin: a potential biomarker of lupus nephritis disease activity. Lupus 25:1230–1236
Tahghighi F, Ziaee V, Moradinejad MH, Rezaei A, Harsini S, Soltani S, Sadr M, Mahmoudi M, Aghighi Y, Rezaei N (2015) Tumor necrosis factor-alpha single nucleotide polymorphisms in juvenile systemic lupus erythematosus. Hum Immunol 76:533–536
Lin YJ, Chen RH, Wan L, Sheu JC, Huang CM, Lin CW, Chen SY, Lai CH, Lan YC, Hsueh KC, Tsai CH, Lin TH, Huang YM, Chao K, Chen DY, Tsai FJ (2009) Association of TNF-α gene polymorphisms with systemic lupus erythematosus in Taiwanese patients. Lupus 18:974–979
Angelo HD, da Silva HA, Asano NM, Muniz MT, de Mascena Diniz Maia M, de Souza PR (2012) Tumor necrosis factor alpha promoter polymorphism 308G/a in Brazilian patients with systemic lupus erythematosus. Hum Immunol 73:1166–1170
Arora V, Verma J, Marwah V, Kumar A, Anand D, Das N (2012) Cytokine imbalance in systemic lupus erythematosus: a study on northern Indian subjects. Lupus 21:595–603
Sinicato NA, Postal M, Peres FA, Pelicari Kde O, Marini R (2014) Dos Santos Ade O, Ramos CD, Appenzeller S (2014) Obesity and cytokines in childhood-onset systemic lupus erythematosus. J Immunol Res 2014:162047. https://doi.org.10.1155/2014/162047
Iida H, Seifert R, Alpers CE, Gronwald RG, Phillips PE, Pritzl P, Gordon K, Gown AM, Ross R, Bowen-Pope DF, Johnson RK (1991) Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci USA 88:6560–6564
Ostendorf T, Kunter U, Grone HJ, Bahlmann F, Kawachi H, Shimizu F, Koch KM, Janjic N, Floege J (2001) Specific antagonism of PDGF prevents renal scarring in experimental glomerulonephritis. J Am Soc Nephrol 12:909–918
Floege J, Eng E, Young BA, Alpers CE, Barrett TB, Bowen-Pope DF, Johnson RJ (1993) Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest 92:2952–2962
Isaka Y, Fujiwara Y, Ueda N, Kaneda Y, Kamada T, Imai E (1993) Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest 92:2597–2601
Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD (1994) Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 263:1759–1762
Papoff G, Cascino I, Eramo A, Starace G, Lynch DH, Ruberti G (1996) An N-terminal domain shared by Fas/Apo-1 (CD95) soluble variants prevents cell death in vitro. J Immunol 156:4622–4630
Misra R, Gupta R (2015) Biomarkers in lupus nephritis. Int J Rheum Dis 18:219–232
Kong KO, Tan AW, Thong BY, Lian TY, Cheng YK, Teh CL, Koh ET, Chng HH, Law WG, Lau TC, Leong KP, Leung BP, Howe HS (2009) Enhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosus. Clin Exp Immunol 156:134–140
El-Gohary A, Hegazy A, Abbas M, Kamel N, Nasef SI (2016) Serum and urinary interferon-gamma-inducible protein 10 in lupus nephritis. J Clin Lab Anal 30:1135–1138
Rose T, Grützkau A, Hirseland H, Huscher D, Dähnrich C, Dzionek A, Ozimkowski T, Schlumberger W, Enghard P, Radbruch A, Riemekasten G, Burmester GR, Hiepe F, Biesen R (2013) IFNα and its response proteins, IP-10 and SIGLEC-1, are biomarkers of disease activity in systemic lupus erythematosus. Ann Rheum Dis 72:1639–1645
Abujam B, Cheekatla S, Aggarwal A (2013) Urinary CXCL-10/IP-10 and MCP-1 as markers to assess activity of lupus nephritis. Lupus 22:614–623
Fu Q, Chen X, Cui H, Guo Y, Chen J, Shen N, Bao C (2008) Association of elevated transcript levels of interferon-inducible chemokines with disease activity and organ damage in systemic lupus erythematosus. Arthritis Res Ther 10:R112
Li H, Ding G (2016) Elevated serum inflammatory cytokines in lupus nephritis patients, in association with promoted hsa-miR-125a. Clin Lab 62:631–638
Segerer S, Banas B, Wörnle M, Schmid H, Cohen CD, Kretzler M, Mack M, Kiss E, Nelson PJ, Schlöndorff D, Gröne HJ (2004) CXCR3 is involved in tubulointerstitial injury in human glomerulonephritis. Am J Pathol 164:635–649
Lu J, Kwan BC, Lai FM, Choi PC, Tam LS, Li EK, Chow KM, Wang G, Li PK, Szeto CC (2011) Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis. Nephrology (Carlton) 16:426–432
Acknowledgements
This study was funded by the National Natural Science Foundation of China (81273314, 81571605) and Shanghai Municipal Education Commission (14ZZ105). We also would like to thank the patients and their families who participated in the study for their time and effort.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content and all authors approved the final version to be published. Tong-xin Chen, Lan-fang Cao and Wei Zhou were responsible for study conception and design. Chen-xing Zhang and Li Cai were responsible for the experiment as well as the analysis and interpretation of data. Kang Shao and Jing Wu were responsible for the acquisition of data. Tong-xin Chen reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Shanghai Children’s Medical Center and all participants signed a consent form in order to participate.
Conflicts of interest
The authors declare that they have no competing interests and all authors read and approved the final manuscript and the final version to be published.
Rights and permissions
About this article
Cite this article
Zhang, Cx., Cai, L., Shao, K. et al. Serum IP-10 is useful for identifying renal and overall disease activity in pediatric systemic lupus erythematosus. Pediatr Nephrol 33, 837–845 (2018). https://doi.org/10.1007/s00467-017-3867-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3867-1