Abstract
Background
Many causes for neonatal hypertension in premature infants have been described; however in some cases no etiology can be attributed. Our objectives are to describe such cases of unexplained hypertension and to compare hypertensive infants with and without chronic lung disease (CLD).
Methods
We reviewed all cases of hypertension in premature infants referred from 18 hospitals over 16 years. Inclusion criteria were hypertension occurring at <6 months of age and birth at <37 weeks gestation; the main exclusion criterion was known secondary hypertension. Continuous variables were compared using analysis of variance. Nominal variables were compared using chi-square tests.
Results
A total of 97 infants met the inclusion criteria, of whom 37 had CLD. Among these infants, hypertension presented at a mean of 11.3 ± 3.2 chronological weeks of age and a postmenstrual age of 39.6 ± 3.6 weeks. Diagnostic testing was notable for plasma renin activity (PRA) being <11 ng/mL/h in 98% of hypertensive infants. Spironolactone was effective monotherapy in 51 of 56 cases of hypertension. Hypertension resolved in all infants, with an average treatment duration of 25 weeks. Significant differences between the two groups of infants were a 0.4 kg lower birthweight and a 2.5 weeks younger gestational age at birth in those with CLD (p < 0.01, p < 0.01, respectively). Hypertension presented in those with CLD 1.8 weeks later, but at the same postmenstrual age as those without CLD (p < 0.01, p = 0.45, respectively).
Conclusion
Premature infants with unexplained hypertension, with and without CLD, presented at a postmenstrual age of 40 weeks with low PRA, transient time course, and a favorable response to spironolactone treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension in premature infants has been recognized since the 1970s. Cases at that time were often associated with high plasma renin activity (PRA) from renal artery thromboembolism related to an umbilical artery catheter [1, 2]. Since then, many causes of hypertension have been reported for this age group [3,4,5,6], but in 8–71% of cases, the cause of hypertension could only be described as cause not identified [7], idiopathic [8, 9], or cause not determined [10]. In the search for causation, many studies have examined risk factor associations using both univariate and multivariate analyses [5, 7, 10,11,12]. Chronic lung disease (CLD) is one such association which is well-documented [5, 7, 10, 13,14,15,16]. Although CLD has appeared on lists of possible causes of neonatal hypertension, a proven mechanism of hypertension due to CLD remains elusive [17,18,19]. Throughout the analysis reported in this review, we consider all of these premature infants without definite secondary cause for hypertension to have unexplained hypertension.
With this paper, we seek to further characterize premature infants with unexplained hypertension, describing common clinical features to aid in future recognition and treatment. The primary objective of this report is to describe the clinical features of unexplained hypertension in premature infants in terms of demographics, disease characteristics, and treatment. The secondary objective is to compare premature infants with unexplained hypertension with and without CLD to see if these groups are similar or different in their presentation, response to treatment, or resolution of hypertension.
Materials and methods
A retrospective chart review was accomplished by identifying all cases of hypertension in premature infants seen within three referral centers located in the northwestern region of the USA over the last 16 years. Institutional review board approval was obtained. Relevant medical records were identified by querying for the diagnosis of hypertension in the discharge diagnosis list or the problem list. Records searched included those born in, transferred into, or seen for consultation at any of the referral centers.
Inclusion criteria for this study were premature birth, defined as a gestational age of <37 weeks at birth, as well as a hypertension diagnosis before age 6 months. Exclusion criteria were evidence of secondary hypertension and inadequate follow-up data which would preclude determination of anti-hypertensive therapy duration. Using the classification categories proposed by Flynn and the expert chart review, we assigned cases to categories of secondary hypertension, including renovascular, congenital and acquired renal parenchymal disease, pulmonary (CLD), cardiac (thoracic coarctation of the aorta), endocrine, medications/intoxications, neoplasia, neurologic, and miscellaneous [6]. Patients with simple cysts or mild pelviectasis detected upon ultrasonography were not included in the renal parenchymal category, as these findings have not been shown to cause hypertension. Patients with prior history of corticosteroid administration, but not at the time of diagnosis, were not included in the medication category.
All patients placed in these categories of secondary hypertension were excluded from the final analysis, with the exception of those with CLD, which we included by design. These patients, who had CLD but no other known cause of hypertension, are referred to here as the CLD group. The remaining patients, in whom no other secondary cause for hypertension was noted, are referred to in the following text as the non-CLD group. The presence of CLD was based on the Vermont Oxford convention of needing oxygen after 36 weeks postmenstrual age [20].
The diagnosis of hypertension was based on the clinical judgment of the attending physician—not based on any specific blood pressure criteria. The date of diagnosis of hypertension was defined as the date on which the attending physician or consulting nephrologist made the diagnosis of hypertension in the medical record, whichever came first. Blood pressure at diagnosis was recorded as the average of all available blood pressure measurements obtained on the day of diagnosis. Blood pressures were also recorded for the day of discharge from the newborn intensive care unit (NICU), the first outpatient visit, the last outpatient visit while still on anti-hypertensive medication, and a follow-up visit when no longer on such medication. The systolic, mean, and diastolic blood pressures were compared to the 95th percentiles for blood pressure as reported in a normative data set compiled by Dionne et al. in 2012 [21]. This data set provides stratified blood pressures by postmenstrual age and was derived from a combination of sources [22,23,24,25,26,27,28].
All inpatient blood pressure measurements were obtained using the oscillometric method and included both arm and leg measurements. For outpatients, systolic and diastolic blood pressure measurements were obtained with sphygmomanometry, with mean blood pressure calculated using the common convention of one-third of the sum of two times diastolic pressure plus systolic pressure. A Doppler device was used for systolic blood pressure measurement when an auscultatory measurement could not be successfully obtained.
Demographic data collected included race, gender, gestational age at birth, birth weight, and history of maternal receipt of antenatal corticosteroids. Data collected from the time of diagnosis included chronologic age, postmenstrual age, blood pressure as described above, presence of any of the above-mentioned exclusion criteria, basic serum chemistry levels, PRA, serum aldosterone level, renal ultrasound results, echocardiogram results, presence of CLD, and history of any exposure to corticosteroids, including human milk exposure. We also determined which infants had umbilical arterial catheters during their NICU stay and calculated the number of days between removal of the catheter and the diagnosis of hypertension. PRA and serum aldosterone testing were performed in one of four referral laboratories, with normal values for each laboratory shown in Table 1. Twenty-one urine cortisol/cortisone ratios were obtained at varying time points in relation to the diagnosis of hypertension in 16 patients. In order to determine if our cohort was exposed to corticosteroids more or less frequently than the overall population, their characteristics were compared with a national clinical database of premature infants [29].
Treatment data collected included the sequence of medications used for the treatment of hypertension, postmenstrual age at start and completion of anti-hypertensive medication, and duration of medication use. The reasons for changes in the medication regimen were recorded. For a subset of 18 patients for whom complete daily blood pressure data were available at onset, diagnosis, and treatment initiation, we calculated the average interval of time between onset and diagnosis of hypertension, average time to blood pressure normalization, and medication needed to bring systolic blood pressure below the 95th percentile for age and gestation. Onset of hypertension was defined as the first day on which the daily average of systolic blood pressure was above the 95th percentile and subsequently stayed above the 95th percentile for more than 2 days. Finally, all charts were examined for evidence of any recurrence of hypertension between the time of resolution and January 2017.
Data were analyzed for all infants included in the study, both combined and separately for those diagnosed with CLD and those without CLD. Tests for significant difference were performed with analysis of variance for continuous variables and with chi-squared tests for nominal variables. A 0.05 significance level was used throughout this analysis.
Results
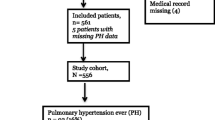
A total of 138 infants originating from 16 different NICU centers and 2 newborn nurseries were identified who met the study inclusion criteria. Of this group, 31 were excluded for known causes of hypertension, including three for renovascular disease, eight for chronic and five for acute renal parenchymal disease, ten for receiving corticosteroids at diagnosis, four for receiving oral sodium supplements at diagnosis, and one for hydrocephalus. A further ten infants were excluded for being lost to follow-up during treatment, thereby preventing characterization of their disease course.
The characteristics of the remaining 97 infants are shown in Table 2. Individual data for the cohort are provided in the electronic supplementary material (ESM 2). Of these 97 infants, 37 were categorized with CLD and 60 without CLD. Table 3 shows the comparison of characteristics between the CLD and non-CLD groups. The only significant between-group differences were a 0.4 kg lower birthweight and a 2.5 weeks earlier gestational age at birth in infants with CLD (p < 0.01, p < 0.01, respectively).
Laboratory and diagnostic imaging data are also presented in Table 2, with comparison between groups shown in Table 3. Very few abnormal values of serum electrolytes, or creatinine, were found in either group. PRA data were obtained for 86 infants; in almost all cases the PRA level was remarkably low, and in 33 infants, PRA levels were below the assay detection limit. Of all PRA levels measured, 98% were below the lower limit of normal for premature infants, or 92% if normal values for term infants are used. For those seven patients for whom the PRA was not low based on infant norms, all but one were receiving diuretic therapy when the PRA levels were obtained, a therapy known to elevate PRA. Serum aldosterone levels were within norms for premature infants in almost all cases. Urine cortisol/cortisone levels were 0.06 ± 0.02 at the time of diagnosis of hypertension, 0.09 ± 0.06 during treatment, and 0.11 ± 0.07 after resolution of the hypertension. There was no difference in these ratios between the CLD and non-CLD groups.
With respect to diagnostic imaging, minor abnormalities were noted in 14 of the 74 patients for whom renal ultrasound data were available, including six with mild pelviectasis and eight with mild nephrocalcinosis. Left ventricular hypertrophy (LVH) was noted in 14 infants and left ventricular dysfunction was noted in two of the 67 infants for whom echocardiograms were obtained near the time of diagnosis of hypertension. LVH was found in 25% of the non-CLD group, but only in 4.3% of the CLD group, approaching but not reaching a significant difference (p < 0.09).
Time course of hypertension presentation and treatment
The diagnosis of hypertension in all infants included in the study was made at 11.3 ± 3.2 weeks of age and 39.6 ± 3.6 weeks postmenstrual age. Of these 97 infants, 75 presented hyptertension before NICU discharge (Table 2). As shown in Table 3, those with CLD were diagnosed with hypertension 1.8 weeks later than those without CLD by chronological age, p < 0.01, but they did not differ with respect to postmenstrual age at diagnosis (39.1 ± 2.5 vs. 39.8 ± 3.4 weeks; p = 0.34).
Blood pressures at diagnosis are shown in Table 4. In all cases, the systolic blood pressure on the date of diagnosis was at or above the 95th percentile for postmenstrual age. Only 59% of mean blood pressures and 43% of diastolic blood pressures were above the 95th percentile at diagnosis of hypertension. No difference was found between CLD or non-CLD groups for systolic (p = 0.60), mean (p = 0.61), or diastolic blood pressure (p = 0.45). Duration of treatment was 25 ± 20 weeks and was not different between groups (p = 0.52). All patients were successfully discontinued from anti-hypertensive medication and were considered to have achieved resolution of the hypertension. Blood pressures off medication are shown in Table 4. Individual data for the cohort are shown in the ESM 1 for five key time points. No patients were documented to have hypertension return in any of the three referral centers in this study over the 16-year span of this study.
The treatment regimens used for this study are shown in Table 5. Diuretic therapy was the most frequently used class of medication, with spironolactone being the most commonly used medication. Spironolactone monotherapy was used initially in 56 infants, discontinued in three infants for lack of efficacy, and was included in the final regimen at hypertension resolution in 75 infants. Spironolactone was also used as part of initial combination therapy in 13 other infants. Concern about somewhat elevated serum potassium levels prompted replacement of spironolactone with other medications in four infants, although no electrocardiogram changes or symptoms of hyperkalemia occurred. Both captopril and hydralazine were ineffective as monotherapy in all cases. Calcium channel blockers were used as monotherapy in seven infants, but for six of these therapy was altered to other medications due to poor efficacy. Chlorothiazide use was successful in two of the five infants when used as monotherapy and in five of the seven infants when used in combination with other medications. Spironolactone in combination with chlorothiazide or hydrochlorothiazide was the most successful combination therapy.
Close examination of the 18 infants for whom daily blood pressure and medication dosing were available showed that the interval between onset and chart diagnosis of hypertension was 14.2 ± 7.8 days. The time required to normalize systolic blood pressure below the 95th percentile was 2.3 ± 1.0 days for spironolactone monotherapy and 3.5 ± 2.6 days for all patients. The average dose of spironolactone at normalization of systolic blood pressure was 2.4 mg/kg/day.
Discussion
This evaluation of 97 premature infants with heretofore unexplained hypertension revealed common features in certain laboratory diagnostics, clinical time course of the high blood pressure, and response to treatment. The infants nearly universally had low PRA levels and normal serum sodium, potassium and aldosterone levels. A majority of patients responded well to spironolactone treatment, often as monotherapy. Hypertension presented at around 40 weeks’ postmenstrual age and resolved within another 25 weeks. Resolution of hypertension occurred in all patients.
This study included 37 infants with CLD and 60 without CLD, and the results demonstrate that there was little difference between the CLD and non-CLD groups in all areas other than gestational age at birth and birthweight. The finding that those with CLD were born earlier and at lower birthweight is not unexpected given the known association of CLD with more extreme prematurity [30].
The blood pressure data from these infants revealed unexplained hypertension to be predominantly systolic, as only 43% of infants meeting systolic criteria also met diastolic criteria for the diagnosis of hypertension. The reason for this discordance is unknown but could be partially due to known difficulty taking manual blood pressures on small infants. Additionally, with the dataset we used to categorize hypertension, diastolic norms for many infants were higher than those for a 1-year-old child using the Fourth Report on the Diagnosis, Evaluation, and Treatment of Hypertension in Children and Adolescents [31].
The consistently low PRA in these infants is a novel finding compared to past reports. Since early reports associating umbilical arterial catheters with hypertension [1, 2], high-renin hypertension has been considered the dominant form of hypertension occurring in premature infants [1, 3, 4, 6]. In 2007 Seliem et al. reported elevated PRA in 33% of hypertensive infants, including all infants for which a cause of hypertension could be identified [12]. The authors did not provided individual PRA levels, but the range of PRA values extended below the lower limit of the infant norm for PRA [12]. There is a paucity of data on PRA in premature infants with or without hypertension with which to compare our results. We noted just two reports of low-renin hypertension in premature infants, describing three infants with suspected monogenic hypertension [32, 33]. Indeed, low-renin hypertension in older children is very often due to genetic errors in steroidogenesis pathways [34, 35]. Our patients lacked both family histories of low-renin hypertension and persistence of hypertension, arguing against a genetic cause of hypertension in our cohort [36].
There are key differences in the regulation of sodium and potassium balance in neonatal or premature kidneys as compared to that in older infants. Neonatal kidneys have impaired regulation of sodium homeostasis which is manifested by elevated PRA and serum aldosterone [37,38,39]. Premature infants have even higher levels of PRA and plasma aldosterone, and show more sodium wasting than more mature infants [40,41,42,43,44]. Aldosterone is regulated by serum potassium and angiotensin II levels by 3–12 months of age in term infants, but it is seemingly dissociated with potassium and PRA at 2–3 days of age [45]. The lack of correlation between serum potassium and plasma aldosterone concentrations has also been noted in young premature infants [40]. Satlin and Zhou have shown that mechanisms of potassium excretion are not fully developed in the preterm infant [46, 47] which may explain the relative lack of hypokalemia in our patients, and in the few reported premature hypertensive infants with low PRA [32, 33].
The pathophysiology of low-renin hypertension in premature infants involves enhanced sodium retention leading to volume expansion and the low-renin state [34, 35]. Assuming normal renal function, this can be due to increased sodium intake, activation of the mineralocorticoid receptor (MR), or activation of the receptor’s dependent mechanisms that enhance sodium channel or transporter activities. Aldosterone, the principal mineralocorticoid affecting the MR [48], was not elevated in our cohort relative to other premature infants. Cortisol can be elevated in the setting of reduced 11B-hydroxysteroid dehydrogenase type 2 (11B–HSD2) activity, an enzyme which converts cortisol into the less potent cortisone. Animal models have shown that placental injury can lead to early hypertension through this mechanism [49, 50]. Our data on cortisol/cortisone ratios, although not extensive, uniformly show these ratios rising from onset through resolution of the hypertension—opposite of what would be expected if this mechanism was operant in our cohort. Other corticosteroids or agents, such as cadmium, can also activate the MR receptor and its dependent mechanisms as well [51, 52]. Multiple sources of exogenous steroid exposure were considered in our cohort. However, none of these common corticosteroid exposures seem likely to be primary causes of hypertension in our cohort, although indirect or remote effects from these exposures cannot be excluded.
In our cohort, spironolactone was initially used for anti-hypertensive treatment noting the success of others in treating older patients with low-renin hypertension (whether or not aldosterone was elevated) [34, 35]. Most of the infants responded rapidly to spironolactone treatment, often as monotherapy in doses of 2.5–3 mg/kg/day. Combination therapy, particularly when spironolactone was paired with a thiazide drug, was also very effective and reduced the concern for hyperkalemia. Other anti-hypertensive agents also appeared to be less effective as monotherapy; however due to early success using spironolactone, limited numbers of patients were treated with other agents, precluding further conclusions on optimal treatment regimens without further study. Very little data have been published on the efficacy of diuretics in treating neonatal hypertension [11].
Our entire cohort showed resolution of hypertension, similar to studies reporting on CLD patients or hypertensive infants presenting after NICU discharge [8, 16]. Other studies did not report complete resolution, but had incomplete follow-up [7, 9] or included patients with renal injury for which resolution might not be expected [12]. Long-term follow-up may be warranted given the connection with premature birth and hypertension later in life [53, 54].
The strengths of the study include being both large and multi-centered, with patients coming from 18 different hospitals, thereby avoiding potential single-center or laboratory bias. The prolonged follow-up period and the ability to detect potential recurrences of hypertension using our shared electronic medical record are also strengths. The lack of a control group and the retrospective nature are weaknesses of the study, suggesting the need for future case-controlled or randomized controlled trials to verify and expand on these findings. The demographics of the Northwestern USA most likely also impacted the distribution of race in our study, reducing our ability to generalize findings to other locations with different ethnic distributions.
In conclusion, unexplained hypertension in premature infants appears to be transient in time course with onset at 10–12 weeks of age and 40 weeks’ postmenstrual age, with resolution over the subsequent 25 weeks. These patients present with low PRA levels with normal electrolytes and normal aldosterone levels for age. Most patients appear to respond well to spironolactone, but further trials are needed to clarify optimum treatment. The observed hypertension seems to be due to sodium retention and is not explained by exogenous sodium delivery, excess corticosteroid exposure, or downregulation of 11B–HSD2. Further investigation is warranted into the cause and mechanism of unexplained low-renin hypertension in premature infants.
References
Adelman RD (1978) Neonatal hypertension. Pediatr Clin North Am 25:99–110
Neal WA, Reynolds JW, Jarvis CW, Williams HJ (1972) Umbilical artery catheterization: demonstration of arterial thrombosis by aortography. Pediatrics 50:6–13
Flynn JT (2000) Neonatal hypertension: diagnosis and management. Pediatr Nephrol 14:332–341
Batisky DL (2014) Neonatal hypertension. Clin Perinatol 41:529–542
Kent AL, Chaudhari T (2013) Determinants in neonatal blood pressure. Curr Hypertens Rep 15:426–432
Flynn JT (2012) Hypertension in the neonatal period. Curr Opin Pediatr 24:197–204
Sahu R, Pannu H, Yu R, Shete S, Bricker JT, Gupta-Malhortra M (2013) Systemic hypertension requiring treatment in the neonatal intensive care unit. J Pediatr 163:84–88
Friedman AL, Hustead VA (1987) Hypertension in babies following discharge from a neonatal intensive care unit. A 3 year follow-up. Pediatr Nephrol 1:30–34
Sheftel DN, Hustead V, Friedman A (1983) Hypertension screening in the follow-up of premature infants. Pediatrics 71:763–766
Singh HP, Hurley RM, Myers TF (1992) Neonatal hypertension. Incidence and risk factors. Am J Hypertens 5:51–55
Blowery DL, Duda PJ, Stokes P, Hall P (2011) Incidence and treatment of hypertension in the neonatal intensive care unit. J Am Soc Hypertens 5:478–483
Seliem WA, Falk MC, Shadbolt B, Kent AL (2007) Antenatal and postnatal risk factors for neonatal hypertension and infant follow-up. Pediatr Nephrol 22:2081–2087
Smets K, Vanhaesebrouck P (1996) Dexamethasone associated systemic hypertension in low birth weight babies with chronic lung disease. Eur J Pediatr 155:573–575
Abman SH, Warady BA, Lum GM, Koops BL (1984) Systemic hypertension in infants with bronchopulmonary dysplasia. J Pediatr 104:929–931
Alagappan A, Mallow MH (1998) Systemic hypertension in very low-birth weight infants with bronchopulmonary dysplasia: incidence and risk factors. Am J Perinatol 15:3–8
Anderson AH, Warady BA, Daily DK, Johnson JA, Thomas MK (1993) Systemic hypertension in infants with severe bronchopulmonary dysplasia: associated clinical factors. Am J Perinatol 10:190–193
Milstein JM, Goetzman BW, Riemenschneider TA, Wennberg RP (1979) Increased systemic vascular resistance in neonates with pulmonary hypertension. Am J Cardiol 44:1159–1162
Cohen G, Lagercrantz H, Katz-Salamon M (2007) Abnormal circulatory stress response of preterm graduates. Pediatr Res 61:329–334
Greenough A, Emery EF, Gamsu HR (1992) Dexamethasone and hypertension in preterm infants. Eur J Pediatr 151:134–135
Horbar JD, Soll RF, Edwards WH (2010) The Vermont Oxford Network: a community practice. Clin Perinatol 37:29–47
Dionne JM, Abitbol CL, Flynn JT (2012) Hypertension in infancy: diagnosis, management and outcome. Pediatr Nephrol 27:17–32
deSwiet M, Fayers P, Shineborne EA (1980) Systolic blood pressure in a population of infants in the first year of life: the Brompton study. Pediatrics 65:1028–1035
Pejovic B, Peco-Antic A, Marinkovic-Eric J (2007) Blood pressure in non-critically ill preterm and full-term neonates. Pediatr Nephrol 22:249–257
Kent AL, Kecskes Z, Shadbolt B, Falk MC (2007) Normative blood pressure data in the early neonatal period. Pediatr Nephrol 22:1335–1341
Kent AL, Meskell S, Falk MC, Shadbolt B (2009) Normative blood pressure data in the early neonatal period. Pediatr Nephrol 24:141–146
Lurbe E, Garcia-Vicent C, Torro I, Fayos JL, Aguilar F, de Llano JM, Fuertes G, Redón J (2007) First-year blood pressure increase steepest in low birthweight newborns. J Hypertens 25:81–86
Kent AL, Kecskes A, Shadbolt B, Falk MC (2007) Blood pressure in the first year of life in healthy infants born at term. Pediatr Nephrol 22:1743–1749
Zubrow AB, Hulman S, Kushner H, Falkner B (1995) Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. J Perinatol 15:470–479
Ellsbury DL, Clark RH, Ursprung R, Handler DL, Dodd ED, Spitzer AR (2016) A multifaceted approach to improving outcomes in the NICU: the Pediatrix 100 000 Babies Campaign. Pediatrics 137(4). doi:10.1542/peds.2015-0389
Eber E, Zach MS (2001) Long-term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax 56:317–323
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114 [2 Suppl 4th Report]:555–576
Field ML, Roy S, Stapleton FB (1985) Low-renin hypertension in young infants. Am J Dis Child 139:823–825
Malagon-Rogers M (2003) Non-glucocorticoid-remediable aldosteronism in an infant with low-renin hypertension. Pediatr Nephrol 19:235–236
New MI, Levine LS (1980) Hypertension of childhood with suppressed renin. Endocr Rev 1:421–430
DiMartino-Nardi J, New MI (1987) Low-renin hypertension of childhood. Pediatr Nephrol 1:99–108
Vehaskari VM (2009) Heritable forms of hypertension. Pediatr Nephrol 24:1929–1937
Kotchen TA, Strickland AL, Rice TW, Walters DR (1972) A study of the renin–angiotensin system in newborn infants. J Pediatr 80:938–946
Dillon MJ, Gillin ME, Ryness JM, deSwiet M (1976) Plasma renin activity and aldosterone concentration in the human newborn. Arch Dis Child 51:537–540
Martinerie L, Pussard E, Foix-L’Helias L, Petit F, Cosson C, Boileau P, Lombes M (2009) Physiological partial aldosterone resistance in human newborns. Pediatr Res 66:325–328
Bourchier D (2005) Plasma aldosterone levels in the 1st week of life in infants less than 30 weeks gestation. Eur J Pediatr 164:141–145
Leslie GI, Barr PA, Gallery EDM, Gyory AZ (1984) Role of renin and aldosterone in establishment of electrolyte balance in very low birthweight neonates. Aust Paediatr J 20:209–212
Stephenson TJ, Pipkin FB, Elias-Jones AC (1991) Factors influencing plasma renin and renin substrate in premature infants. Arch Dis Child 66:1150–1154
Martinerie L, Pussard E, Yousef N, Cosson C, Lema I, Husseini K, Mur S, Lombès M, Boileau P (2015) Aldosterone-signaling defect exacerbates sodium wasting in very preterm neonates: the Premaldo Study. J Clin Endocrionol Metab 100:4074–4081
Sulyok E, Németh M, Tényi I, Csaba I, Györy E, Ertl T, Varga F (1979) Postnatal development of renin–angiotensin–aldosterone system, RAAS, in relation to electrolyte balance in premature infants. Pediatr Res 13:817–820
Siegler RL, Crouch RH, Hackett TN, Walker M, Jubiz W (1977) Potassium-renin-aldosterone relationships during the first year of life. J Pediatr 91:52–55
Zhou H, Satlin LM (2004) Renal potassium handling in healthy and sick newborns. Semin Perinatol 28:103–111
Satlin LM (1999) Regulation of potassium transport in the maturing kidney. Semin Nephrol 19:155–165
Rozansky DJ (2006) The role of aldosterone in renal sodium transport. Semin Nephrol 26:173–181
Murotsuki J, Gagnon R, Pu X, Yang K (1998) Chronic hypoxemia selectively down-regulates 11B-hydroxysteroid dehydrogenase type 2 gene expression in the fetal sheep kidney. Biol Reprod 58:234–239
Kajantie E, Dunkel L, Turpeinen U, Stenman U, Wood P, Nuutila M, Andersson S (2003) Placental 11B-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab 88:493–500
Stewart PM, Valentino R, Wallace AM, Burt D, Shackleton CH, Edwards CR (1987) Mineralocorticoid activity of liquorice: 11B-hydroxysteroid deficiency comes of age. Lancet 1:821–824
Walker BR, Aggarwal I, Stewart PM, Padfield PL, Edwards CR (1995) Endogenous inhibitors of 11B-hydroxysteroid dehydrogenase in hypertension. J Clin Endocrinol Metab 80:529–533
Ingelfinger JR, Nuyt A (2012) Impact of fetal programming, birth weight, and infant feeding on later hypertension. J Clin Hypertens 14:365–371
Poplawska K, Dudek K, Koziarz M, Cieniawski D, Drożdż T, Smiałek S, Drożdż D, Kwinta P (2012) Prematurity-related hypertension in children and adolescents. Int J Pediatr 212:1–8
Acknowledgments
The authors thank their colleagues in the Pediatric Nephrology and Neonatology Divisions at Oregon Health & Science University and at St. Luke’s Children’s Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Funding
This study was funded by the “Friends of Doernbecher” organization in Portland, Oregon through the Doernbecher Children’s Hospital Foundation, and support also came from the Henry Hillman Jr. Foundation. Neither organization was involved in the design and conduct of the study, in the collection, management, analysis and interpretation of data and in the preparation, review or approval of the manuscript.
Conflict of interest
The authors declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jenkins, R.D., Aziz, J.K., Gievers, L.L. et al. Characteristics of hypertension in premature infants with and without chronic lung disease: a long-term multi-center study. Pediatr Nephrol 32, 2115–2124 (2017). https://doi.org/10.1007/s00467-017-3722-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3722-4