Abstract
Background
Cross-sectional studies of children with prevalent nephrotic syndrome (NS) have shown 25-vitamin D (25(OH)D) deficiency rates of 20–100 %. Information on 25(OH)D status in incident patients or following remission is limited. This study aimed to assess 25(OH)D status of incident idiopathic NS children at presentation and longitudinally with short-term observation.
Methods
Multicenter longitudinal study of children (2–18 years old) from 14 centers across the Midwest Pediatric Nephrology Consortium with incident idiopathic NS. 25(OH)D levels were assessed at diagnosis and 3 months later.
Results
Sixty-one children, median age 5 (3, 11) years, completed baseline visit and 51 completed second visit labs. All 61 (100 %) had 25(OH)D < 20 ng/ml at diagnosis. Twenty-seven (53 %) had 25(OH)D < 20 ng/ml at follow-up. Fourteen (28 %) children were steroid resistant. Univariate analysis showed that children prescribed vitamin D supplements were less likely to have 25(OH)D deficiency at follow-up (OR 0.2, 95 % CI 0.04, 0.6). Steroid response, age, and season did not predict 25(OH)D deficiency. Multivariable linear regression modeling showed higher 25(OH)D levels at follow-up by 13.2 ng/ml (SE 4.6, p < 0.01) in children supplemented with vitamin D.
Conclusions
In this incident idiopathic NS cohort, all children at diagnosis had 25(OH)D deficiency and the majority continued to have a deficiency at 2–4 months. Supplemental vitamin D decreased the odds of 25(OH)D deficiency at follow-up, supporting a role for supplementation in incident NS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic nephrotic syndrome (NS) is one of the most common forms of kidney disease in children, affecting 15 to 20 per 100,000 children, often with a frequently remitting and relapsing course [1, 2]. In studies of pediatric patients with prevalent primary and secondary NS, 25-hydroxyvitamin D (25(OH)D) deficiency ranges from 20 to 100 % in patients who were surveyed at different points during their disease [3–7], which contrasts with rates of 9–18 % in the general pediatric population [8–10]. Initially, these abnormalities were thought to be transient in nature and resolved with remission, but recent data suggests that 25(OH)D deficiency persists despite achieving remission [11].
Abnormal vitamin D metabolism in idiopathic NS is multi-factorial, with contributions from losses of both vitamin D binding protein and 25(OH)D in the urine [12, 13]. The urinary losses of vitamin D binding protein may be secondary to proteinuria, overwhelming the proximal tubule reabsorption via megalin and cubilin pathways [14]. Deficiency in 25(OH)D may lead to hypocalcemia, hyperparathyroidism, and diminished bone mineral density/content. Vitamin D deficiency has also been associated with multiple systemic effects including elevated blood pressure [15, 16], metabolic syndrome, cardiovascular disease [17], anemia [18], and impaired immune system regulation [19].
Complicating the impact of 25(OH)D deficiency on the bones in children with NS is the repeated exposure to glucocorticoids. The initial treatment at diagnosis and with each relapse exceeds the 5 mg/day of prednisone shown to cause osteoporosis in adults [20]. This has led to an increased interest in the impact of pediatric NS on the bone metabolism in developing children. Children with NS have been shown to have decreased bone mineralization, although the mechanism was not defined [21, 22]. Furthermore, studies in adult survivors of steroid-sensitive minimal change disease show persistent bone abnormalities [23]. These findings have led to an increased interest in differing strategies and trials around supplementation of calcium and vitamin D in children with NS [24–26].
There is a significant gap in the knowledge about 25(OH)D levels in patients with incident NS and the effect of disease course on these levels following initial treatment. To date, there has not been a study evaluating 25(OH)D deficiency in children with prospective observation beginning with the initial diagnosis of NS.
This multicenter international prospective longitudinal study aimed to: (1) describe the prevalence of 25(OH)D deficiency at presentation with NS, (2) describe the prevalence of continued 25(OH)D deficiency at 3 months following initial treatment, and (3) to investigate the association of treatment response and supplementation with 25(OH)D deficiency at follow-up. We hypothesized that 25(OH)D deficiency would be common at presentation and at 3-month follow-up in children with incident NS and that steroid response pattern and supplementation with vitamin D would predict deficiency at follow-up.
Methods
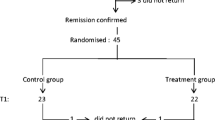
We performed a prospective observational study of children recruited from the participating centers of the Midwest Pediatric Nephrology Consortium with incident NS. This study enrolled 61 pediatric patients from 14 participating member institutions in North America (see author affiliations). The inclusion criteria were age 2–18 years, incident NS with ≤ 14 days of corticosteroid treatment, urine protein/creatinine > 2 mg/mg or urinalysis ≥ 2 + protein and edema. We excluded patients with evidence of a disease process other than idiopathic NS, including hypocomplementemia, positive anti-nuclear antibody (>1:80) or systemic vasculitis.
Enrolled children underwent two study visits. The first study visit was within 2 weeks of corticosteroid initiation. The second study visit was 2–4 months following enrollment, which was timed to coincide with the completion of a standard 12-week corticosteroid treatment for new-onset idiopathic NS. The treatment regimen was at the discretion of the treating physician. At each visit, data collection included race, season, geographical location, medications, response to therapy, and local laboratory data when available (including electrolytes and urine). Relapse, remission, and steroid dependence vs. resistance were defined according to published standards [2]. The seasons were defined as winter (12/21–3/20), spring (3/21–6/20), summer (6/21–9/20), and fall (9/21–12/20). Patient locations in Michigan, Ohio, Ontario, Iowa, Missouri, Wisconsin, and Utah were classified as North. Patient locations in North Carolina, South Carolina, Kentucky, Georgia, and Louisiana were classified as South. Intact parathyroid hormone (iPTH), 25(OH)D and 1,25-hydroxyvitamin D (1,25(OH)D) were obtained at both study visits and measured at a central laboratory.
Blood samples were frozen at −80 °C and shipped to the clinical laboratory at the University of Michigan. The 25(OH)D levels were measured using a DiaSorin chemiluminescence assay with a laboratory reference range of 25–100 ng/ml. The 1,25(OH)D levels were measured using the DiaSorin radioimmunoassay with normal range of 18–72 pg/ml. Intact PTH (iPTH) levels were assessed using immunometric chemiluminescence with normal values of 10–65 pg/ml. There were inadequate samples for analysis of iPTH (one baseline; one return visit) and 1,25 (OH) D (seven baseline; five return visit). When local electrolytes were not available, samples were run centrally. The vitamin D test results were reported to the providers who were subsequently allowed to modify patient care at their discretion.
Outcome
The primary outcome of interest was vitamin D deficiency as measured by the 25(OH)D levels in the blood, which is reflective of total body stores [27]. The analysis was performed utilizing two cut-offs for inadequate 25(OH)D levels < 20 ng/ml and < 30 ng/ml [8, 10].
Statistical analysis
Characteristics of baseline and follow-up samples were described using median (inter-quartile range) for continuous variables and frequency (percentage) for categorical variables. The relationship between 25(OH)D, 1,25(OH)2D and iPTH at baseline and follow-up were tested using Pearson correlation coefficients. Possible predictors of the two dichotomous outcome variables (25(OH)D < 20 ng/ml and <30 ng/ml) were assessed using an unconditional binary logistic regression. In a separate analysis, we tested for differences in 25(OH)D levels as a continuous variable using linear regression. We tested the following covariates: age, sex, season, race, location (North vs. South), steroid response pattern, and vitamin D supplementation. All analyses were conducted using SAS software (SAS Institute Inc. Cary, NC, USA).
Results
Sixty-one patients with incident NS were enrolled and completed the baseline study visit. The median age at enrollment was 5 (3, 11) years. Twenty-one patients (34 %) were enrolled in the winter and 17 patients (28 %) were enrolled from states in the North. Seventeen patients (28 %) were taking prednisone and four patients (7 %) were taking vitamin D supplements at study enrollment. The median 25(OH)D level at enrollment was 9 (7, 11) ng/ml. All 61 patients had 25(OH)D levels < 20 ng/ml at enrollment. Twenty-eight (47 %) of 60 patients with measured iPTH values had an elevated iPTH level (>65 pg/ml) at the baseline visit. Nineteen (35 %) of 54 patients with measured 1,25(OH)D level had a low 1,25(OH)D level (<18 pg/ml) at the baseline visit. There was no correlation between corrected calcium and 25(OH)D, 1,25(OH)D or iPTH at baseline. Table 1 summarizes patient demographics and laboratory characteristics at enrollment.
Fifty-one children completed the second visit (84 % retention rate). The median time between visits was 2.6 (2.1, 3.4) months. The second visit most commonly occurred in the summer (n = 17, 33 %). Fourteen patients (28 %) were steroid resistant at the follow-up visit and 16 patients were in relapse at follow-up. The median 25(OH)D level at follow-up was 19 (10, 25) ng/ml. Seventeen patients (33 %) were receiving vitamin D supplementation at follow-up. Twenty-seven patients (53 %) had 25(OH) D levels < 20 ng/ml and 43 patients (84 %) had 25(OH) D levels < 30 ng/ml. Fifteen (30 %) of 50 patients with measured iPTH values had an elevated intact PTH level (>65 pg/ml) at the follow-up. One patient (2 %) of 46 patients with measured 1,25(OH)D level had a low 1,25(OH)D level (<18 pg/ml) at follow-up. The patient demographics and laboratory characteristics at the follow-up visit are presented in Table 2.
We evaluated the association of 25(OH)D deficiency with abnormalities in 1,25(OH)D and iPTH levels. At baseline, all patients had 25(OH)D deficiency, 47 % had an elevated iPTH level and 35 % had a low 1,25(OH)D level. At baseline there were no significant correlations between 25(OH)D levels and 1,25(OH)D (r = −0.02, p = 0.89) or iPTh levels (r = −0.03, p = 0.81). At follow-up, 50 patients had iPTH values available for evaluation and 27 of these patients (54 %) had 25(OH)D < 20 ng/ml and 43 patients (86 %) had 25(OH) D levels < 30 ng/ml. Elevated iPTH levels were present in 12 patients (44 %) and 14 (33 %) of the patients with 25(OH)D < 20 ng/ml and < 30 ng/ml, respectively. At follow-up, 46 patients had 1,25(OH)D level values measured and 25 of these patients (54 %) had 25(OH)D < 20 ng/ml and 39 patients (85 %) had 25(OH) D levels < 30 ng/ml. Low 1,25(OH)D levels were present in 0 patients and 1 patient with 25(OH)D < 20 ng/ml and < 30 ng/ml, respectively. At follow-up, there were no significant correlations between 25(OH)D levels and 1,25(OH)D (r = 0.13, p = 0.39) or iPTh levels (r = −0.01, p = 0.93). For patients that were in remission at follow-up, the correlation between 25(OH)D levels with 1,25(OH)D (r = 0.33, p = 0.06) and iPTh levels approached significance (r = −0.34, p = 0.05). For patients that were in relapse at follow-up, there was no significant correlation between 25(OH)D levels and 1,25(OH)D (r = −0.17, p = 0.57), but there was a correlation with iPTh levels (r = 0.54, p = 0.03).
Table 3 shows the univariate analysis describing the association between patient characteristics and 25(OH)D deficiency at the follow-up visit as defined by 25(OH)D < 20 ng/ml. When utilizing a definition of < 20 ng/ml, male gender and black race were associated with increased odds of 25(OH)D deficiency. Vitamin D supplementation at follow-up and enrollment from sites in the North were protective against deficiency in our cohort. The median 25(OH) levels were not significantly different between patients that were supplemented with vitamin D and those that were not (p = 0.62). Steroid resistance was not associated with 25(OH)D deficiency. For participants that had a urine protein-to-creatinine ratio available at the second visit (n = 33), the urine protein-to-creatinine ratio was not associated with 25(OH)D deficiency. Children from Northern institutions were more likely to be supplemented with vitamin D (57 vs. 24 %, p = 0.03). Children from Northern institutions were not more likely to have visits in the summer months (p = 0.22).
In multivariable analysis, linear regression modeling showed higher 25(OH)D levels at follow-up by 11.5 ng/ml (SE 4.9, p = 0.02) in children residing in the North (compared to those in the South) and by 13.2 ng/ml (SE 4.6, p < 0.01) in children supplemented with vitamin D (compared to those who were not supplemented). A sensitivity analysis was performed with the addition of serum albumin to the final linear regression model, which showed that the serum albumin level did not predict 25(OH)D levels (β = 3.2, SE 2.3, p = 0.17), but children in the North (β = 10.4, SE 4.9, p = 0.04) and those supplemented with vitamin D (β = 13.4, SE 4.6, p < 0.01) continued to have higher 25(OH)D levels. Factors that were not predictive of 25(OH)D deficiency at the follow-up visit included: 25(OH)D level at the initial visit, age, male, season, black race, and steroid resistance.
Discussion
This is the largest multi-center study to date in North American children with incident idiopathic NS evaluating 25(OH)D deficiency and the first study to prospectively follow 25(OH)D levels from diagnosis in this patient population. Here we demonstrate that 25(OH)D deficiency is universal at diagnosis and 25(OH)D deficiency persists at follow-up in a significant number of patients. We also show that children receiving vitamin D supplementation at follow-up were less likely to have low 25(OH)D levels during that visit.
There has been a long-standing recognition that children with active NS are prone to the development of 25(OH)D deficiency. Freundlich et al. began to explore 25(OH)D deficiency in 1985 in 16 children with active NS, showing levels < 20 ng/ml in all children [4]. This group extended these findings in a cohort of 58 children with prevalent disease followed during relapses and remissions, revealing that children in relapse had a mean 25(OH)D level of 9 ng/ml and during remission these levels improved to a mean of 30 ng/ml [3]. Huang et al. reported similar findings of normalized 25(OH)D levels during remission in 25 children with prevalent NS [28]. These data suggested that 25(OH)D deficiency in this population may be transient. Since that time, there has been an increasing amount of data suggesting that 25(OH)D levels may not completely normalize when children go into remission [6, 11, 29]. In 2005, Weng et al. evaluated 25(OH)D levels in children with NS in remission showing that over 90 % had 25(OH)D < 30 ng/ml and 68 % had levels < 20 ng/ml [6]. Our findings are consistent with the previous literature demonstrating 25(OH)D deficiency in 100 % of our cohort of children with incident NS with a median 25(OH)D level of 9 (7, 11) ng/ml. Furthermore, for the first time we report data obtained from follow-up of the 25(OH)D level prospectively after diagnosis in children with idiopathic NS. We show that abnormalities in 25(OH)D levels persist, with 53 % of children with 25(OH)D levels < 20 mg/dl and 84 % < 30 mg/dl at a median follow-up of 2.6 months after diagnosis.
In the last decade, there has been increased interest in the prevalence of 25(OH)D deficiency in pediatric patients. In recent reports from the National Health and Nutrition Examination Survey (NHANES), the prevalence of 25(OH)D abnormalities in healthy children across America has been assessed. These reports show a high prevalence of 25(OH)D levels < 20 ng/ml of 14–18 % [9, 10]. The rates become even more profound when one considers the prevalence of deficiency with a definition of < 30 ng/ml of 61 % in the general US pediatric population [8]. In this context, our data highlight the persistent abnormalities in 25(OH)D levels in children with NS following diagnosis. Past reports of children with NS in North America were often single center in nature and it was difficult to generalize these findings or compare them to datasets such as NHANES. We extend these findings by providing the most geographically diverse population of children with NS in North America reported to date. Indeed, our data suggest that abnormalities in 25(OH)D levels persist and occur at a greater frequency than would be expected in the general population.
The increased interest in the prevalence of 25(OH)D deficiency in children with NS parallels an increased interest in the impact of NS and its treatment on bone development in these children. This is of particular interest given that a daily dose of systemic steroids as low as 5 mg per day has been shown to contribute to osteoporosis in adults [20]. This is of concern in pediatric patients following a new diagnosis of NS where steroid treatment protocols exceed the dose at which adult osteoporosis risk increases with repeated exposures to steroids during subsequent relapses [1, 2]. Given this information, there has been renewed interest in the impact of childhood NS on the developing bones in children. When appropriate corrections for body size are employed, the literature has been consistent on documenting a negative impact of steroid exposure on bone health in children with NS [21, 22]. Studies utilizing bone biopsy data have demonstrated abnormalities in the bones of children with NS treated with steroids, including increased focal osteomalacia and bone resorption relative to controls [30]. Recently, Choudhary et al. performed an interventional trial in children with new-onset NS, which showed that children with incident disease had abnormalities in bone mineral content at 12 weeks, and supplemental calcium and vitamin D were protective against these bone abnormalities [24]. Unfortunately, this study did not report on the impact supplementation had on 25(OH)D levels. We extend these findings by showing that vitamin D supplementation following diagnosis with new-onset NS is protective against lower 25(OH)D levels at follow-up. This finding persisted irrespective of the definition of 25(OH)D deficiency utilized and accounting for factors classically associated with low 25(OH)D levels in healthy children. Our data suggest that vitamin D supplementation may decrease the risk of ongoing deficiency. Despite this compelling data, caution must be taken, as a number of very important questions remain about supplementation including the optimal form, dose, and duration of vitamin D therapy for these children. Interventional trials are needed to determine if vitamin supplementation is effective at correcting deficiency and improving bone mineral health in children with NS.
In the context of the high rates of 25(OH)D deficiency in children with NS, it is important to evaluate the potential mechanisms and biochemical implications for 25(OH)D deficiency. A known mechanism for the development of 25(OH)D deficiency is the loss of vitamin D-binding protein in the urine during the active phase of NS. This is thought to contribute to the transient nature of 25(OH)D deficiency previously reported in the literature in children with NS. While we did not measure vitamin D-binding protein levels in this study, we did evaluate proteinuria and serum albumin levels at follow-up, which we used as surrogates. The degree of proteinuria and lower serum albumin did not predict 25(OH)D deficiency at follow-up, though the serum albumin level did approach significance. This suggests that the 25(OH)D deficiency seen in children with NS at 3 months following diagnosis cannot simply be explained by persistent disease activity. In evaluating the impact of 25(OH)D deficiency in children with NS, it is important to evaluate the impact on 1,25(OH)D and iPTH levels. An interesting finding in our study was that 35 % of patients had low 1,25(OH)D levels and 47 % had elevated iPTH levels at baseline, despite a rate of 100 % 25(OH)D deficiency. At follow-up, we found that of those with 25(OH)D deficiency, 44 % had elevated iPTH and there were no abnormalities in 1,25(OH)D levels. These findings are consistent with previous reports and extend them by describing this phenomenon in incident patients. Furthermore, abnormalities in bone mineralization have been reported in children with NS despite “normal” 1,25(OH)D and iPTH levels. In evaluating the correlations between 25(OH)D levels and 1,25(OH)D and iPTH, we show that there are no significant correlations at baseline or follow-up when the whole cohort is examined, but there are divergent correlations when patients are dichotomized by remission status. In children in remission at follow-up, there was a marginally significant positive correlation between 25(OH)D level with 1,25(OH)D and a negative correlation with iPTH. This is consistent with the anticipated physiologic response to improved 25(OH) levels. An interesting finding is the positive correlation seen between 25(OH)D and iPTH at follow-up in children in relapse, and warrants further evaluation of potential mechanisms. Further studies evaluating these parameters and their impact in children with NS are warranted.
In reviewing our study in the context of previous studies and epidemiologic data, there are some interesting findings. One such finding is the influence of black race on the rates of 25(OH)D deficiency. In univariate analysis, black race was shown to be strongly associated with 25(OH)D deficiency consistent with NHANES epidemiologic data. However, on linear regression analysis, this association did not persist. This may simply reflect the small sample size or the importance of other factors outside of race. Another interesting finding is the fact that children from “northern” institutions were less likely to have 25(OH)D deficiency. This finding is likely representative of the relatively small distance between some of the sites (North vs. South) coupled with local practice variations in supplementation. Indeed, larger studies are warranted to further clarify the factors that predict 25(OH)D deficiency in these patients.
Our study has a number of strengths, such as the inclusion of a large sample of children with new-onset idiopathic NS from a geographically diverse patient population across North America. To our knowledge, this is the first such longitudinal multicenter study of a North American population evaluating vitamin D in children following diagnosis. Furthermore, we present data on a racially diverse population that is consistent with the patterns of presentation of NS in North American children. The limitations of our study include a short observation period of only 3 months, a modest sample size, and inability to assess causal relationships between vitamin D supplementation and return to normal 25(OH)D levels, as this was an observational study.
Conclusions
We present the largest study to date in North American children with incident idiopathic NS evaluating 25(OH)D deficiency and the first study to prospectively follow 25(OH)D levels after diagnosis in this patient population. In this national sample, all children with incident NS had 25(OH)D deficiency at diagnosis and the majority continued to have a deficiency at 2–4 months. Furthermore, supplemental vitamin D decreased the odds of 25(OH)D deficiency, suggesting the need for future interventional studies addressing vitamin D supplementation in incident NS to evaluate optimal vitamin D dosing and long-term impact in these children.
References
(1978) Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the international study of kidney disease in children. Kidney Int 13:159–165
Gipson DS, Massengill SF, Yao L, Nagaraj S, Smoyer WE, Mahan JD, Wigfall D, Miles P, Powell L, Lin JJ, Trachtman H, Greenbaum LA (2009) Management of childhood onset nephrotic syndrome. Pediatrics 124:747–757
Freundlich M, Bourgoignie JJ, Zilleruelo G, Abitbol C, Canterbury JM, Strauss J (1986) Calcium and vitamin D metabolism in children with nephrotic syndrome. J Pediatr 108:383–387
Freundlich M, Bourgoignie JJ, Zilleruelo G, Jacob AI, Canterbury JM, Strauss J (1985) Bone modulating factors in nephrotic children with normal glomerular filtration rate. Pediatrics 76:280–285
Grymonprez A, Proesmans W, Van Dyck M, Jans I, Goos G, Bouillon R (1995) Vitamin D metabolites in childhood nephrotic syndrome. Pediatr Nephrol 9:278–281
Weng FL, Shults J, Herskovitz RM, Zemel BS, Leonard MB (2005) Vitamin D insufficiency in steroid-sensitive nephrotic syndrome in remission. Pediatr Nephrol 20:56–63
Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB (2009) Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res 24:503–513
Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML (2009) Prevalence and associations of 25-hydroxy vitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124:e362–370
Mansbach JM, Ginde AA, Camargo CA Jr (2009) Serum 25-hydroxy vitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics 124:1404–1410
Saintonge S, Bang H, Gerber LM (2009) Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics 123:797–803
Banerjee S, Basu S, Sengupta J (2013) Vitamin D in nephrotic syndrome remission: a case–control study. Pediatr Nephrol 28:1983–1989
Goldstein DA, Oda Y, Kurokawa K, Massry SG (1977) Blood levels of 25-hydroxy vitamin D in nephrotic syndrome. Studies in 26 patients. Ann Intern Med 87:664–667
Lambert PW, De Oreo PB, Fu IY, Kaetzel DM, von Ahn K, Hollis BW, Roos BA (1982) Urinary and plasma vitamin D3 metabolites in the nephrotic syndrome. Metab Bone Dis Relat Res 4:7–15
Dickson LE, Wagner MC, Sandoval RM, Molitoris BA (2014) The proximal tubule and albuminuria: really! J Am Soc Nephrol 25:443–453
Forman JP, Curhan GC, Taylor EN (2008) Plasma 25-hydroxy vitamin D levels and risk of incident hypertension among young women. Hypertension 52:828–832
Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB (2012) Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. Am J Hypertens 25:1215–1222
Swales HH, Wang TJ (2010) Vitamin D and cardiovascular disease risk: emerging evidence. Curr Opin Cardiol 25:513–517
Lac PT, Choi K, Liu IA, Meguerditchian S, Rasgon SA, Sim JJ (2010) The effects of changing vitamin D levels on anemia in chronic kidney disease patients: a retrospective cohort review. Clin Nephrol 74:25–32
Zold E, Szodoray P, Kappelmayer J, Gaal J, Csathy L, Barath S, Gyimesi E, Hajas A, Zeher M, Szegedi G, Bodolay E (2010) Impaired regulatory T-cell homeostasis due to vitamin D deficiency in undifferentiated connective tissue disease. Scand J Rheumatol 39:490–497
Leonard MB, Zemel BS (2002) Current concepts in pediatric bone disease. Pediatr Clin N Am 49:143–173
Gulati S, Godbole M, Singh U, Gulati K, Srivastava A (2003) Are children with idiopathic nephrotic syndrome at risk for metabolic bone disease? Am J Kidney Dis 41:1163–1169
Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA (2004) Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med 351:868–875
Hegarty J, Mughal MZ, Adams J, Webb NJ (2005) Reduced bone mineral density in adults treated with high-dose corticosteroids for childhood nephrotic syndrome. Kidney Int 68:2304–2309
Choudhary S, Agarwal I, Seshadri MS (2014) Calcium and vitamin D for osteoprotection in children with new-onset nephrotic syndrome treated with steroids: a prospective, randomized, controlled, interventional study. Pediatr Nephrol 29:1025–1032
Bak M, Serdaroglu E, Guclu R (2006) Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr Nephrol 21:350–354
Gulati S, Sharma RK, Gulati K, Singh U, Srivastava A (2005) Longitudinal follow-up of bone mineral density in children with nephrotic syndrome and the role of calcium and vitamin D supplements. Nephrol Dial Transplant 20:1598–1603
Papandreou D, Malindretos P, Karabouta Z, Rousso I (2010) Possible health implications and low vitamin D status during childhood and adolescence: an updated mini review. Int J Endocrinol 2010:472173
Huang JP, Bai KM, Wang BL (1992) Vitamin D and calcium metabolism in children with nephrotic syndrome of normal renal function. Chin Med J (Engl) 105:828–832
Biyikli NK, Emre S, Sirin A, Bilge I (2004) Biochemical bone markers in nephrotic children. Pediatr Nephrol 19:869–873
Freundlich M, Jofe M, Goodman WG, Salusky IB (2004) Bone histology in steroid-treated children with non-azotemic nephrotic syndrome. Pediatr Nephrol 19:400–407
Acknowledgments
The investigators are indebted to the children and families who graciously participated in this study. This work was supported by a grant from the Renal Research Institute. M.E.S. was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from NIH/NCATS, and L40 DK099748 from NIH/NIDDK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
The author(s) declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Each site obtained individual institutional IRB approval. Parents and children gave informed consent and assent respectively.
Rights and permissions
About this article
Cite this article
Selewski, D.T., Chen, A., Shatat, I.F. et al. Vitamin D in incident nephrotic syndrome: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 31, 465–472 (2016). https://doi.org/10.1007/s00467-015-3236-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3236-x