Abstract
Background
Rituximab (RTX) is known to be effective for the treatment of refractory steroid-dependent nephrotic syndrome (SDNS). However, there are insufficient data on the risk factors for relapse and long-term outcome after RTX treatment.
Methods
We administered a single dose of RTX to patients with refractory SDNS from November 2007 to December 2013 and continued with immunosuppressants. The risk factors for early relapse and long-term outcome were analyzed.
Results
Eighty-one patients were included and the observation period was 13–90 months. Seventy-six patients (94 %) discontinued steroids. Median duration of B-cell depletion was 160 days and 50 % relapse-free survival was 482 days. In multivariate analyses, only a history of steroid-resistant nephrotic syndrome (SRNS) was a statistically significant risk factor (hazard ratio, 2.44; p = 0.048). Fifty percent relapse-free survival in patients without a history of SRNS was 615 days, longer than that of patients with one relapse (393 days; p = 0.005). Fifty-one patients (63 %) received additional RTX treatments for relapses. At last observation, patients using calcineurin inhibitors decreased from 89 % to 23 %, and 12 patients (15 %) discontinued immunosuppressants.
Conclusions
Rituximab treatment followed by immunosuppressants is an effective option for patients with SDNS, although a history of SRNS is a risk factor for early relapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with steroid-dependent nephrotic syndrome (SDNS) need immunosuppressive agents (IS) such as cyclosporin A (CsA), tacrolimus (Tac), cyclophosphamide, mizoribine, and mycophenolate mofetil (MMF) to reduce the number of relapses and prevent steroid toxicity. However, in refractory cases, patients continue to suffer from steroid-dependent relapses. Moreover, these IS can have significant adverse effects, such as chronic nephrotoxicity of calcineurin inhibitors [1]. Because of the possibility of gonadotoxicity, cyclophosphamide is recommended to be used within limited cumulative doses [2]. Therefore, even if these IS are effective, it is not feasible to use them for a prolonged period.
Rituximab (RTX) is a chimeric monoclonal antibody directed against the cell surface antigen CD20 expressed on B lymphocytes. It has been proven to be effective for preventing relapses in patients with refractory SDNS [3–11] and was approved for use in Japan on 29 August 2014, to treat childhood-onset refractory SDNS or frequently relapsing nephrotic syndrome [12], although the mechanism has not yet been clarified. However, its effect is usually limited to during B-cell depletion, and patients suffer from relapses after B-cell recovery [3, 4, 7].
As we have previously shown that long-term remission was achieved in SDNS patients with the use of IS after RTX treatment [4, 7], our strategy was to continue IS use (MMF or calcineurin inhibitors) after RTX treatment. However, there have been several reports that assessed the risk factors for relapse and long-term outcome after RTX treatment [12–16]. Here, we evaluated the long-term outcome of 81 patients who were treated with a single dose of rituximab and continued IS. We also analyzed the risk factors for first relapse after initial RTX treatment.
Patients and methods
Inclusion criteria of patients
Patients with childhood-onset refractory SDNS (steroid dependence under IS) who received a single dose of RTX between November 2007 and December 2013 at the National Center for Child Health and Development in Tokyo, Japan, continued with IS after RTX and were followed for more than 1 year, were included in this retrospective study. Patients with a history of steroid-resistant nephrotic syndrome (SRNS) who later acquired steroid sensitivity and SDNS were also enrolled in this study. SDNS was defined as two consecutive relapses during corticosteroid therapy or within 14 days after cessation of therapy; SRNS was defined as the failure to achieve remission despite therapy with prednisolone (PSL) at 60 mg/m2/day for 4 weeks. Relapse was defined as morning proteinuria of 3+ on a dipstick for 3 consecutive days.
Treatment protocol of RTX
Rituximab was administered after remission of nephrotic syndrome at a single dose of 375 mg/m2. To minimize infusion reactions, patients received 1 mg/kg of intravenous methylprednisolone, oral acetaminophen (10 mg/kg, maximum of 300 mg), and chlorpheniramine maleate (0.04 mg/kg, maximum of 2 mg) 30 min before RTX infusions. All patients were admitted to our center and monitored for at least 24 h after RTX infusion in case of any reactions. After RTX treatment, clinical and laboratory parameters, including complete blood counts, biochemical parameters, serum immunoglobulin levels, and CD19+ B-cell counts using flow cytometry were monitored at least once a month until B-cell recovery. B-cell depletion was defined as CD19+ B-cell counts less than 1 % of total lymphocytes, while B-cell recovery was defined as more than 1 %. Steroids were tapered and discontinued after RTX infusions, although the protocol of the discontinuation of steroids was not restricted. IS were continued after RTX treatment. PSL therapy was administered for relapses during the observation period. In the event of relapses after B-cell recovery, additional RTX treatment was indicated.
Outcomes and statistical analysis of risk factors for the relapse
Rate of patients and time of steroid discontinuation, duration of B-cell depletion, relapse rates, and 50 % cumulative remission rates, re-administration of RTX and final outcomes were examined. Survival curve of cumulative remission rate after the initial RTX treatment was analyzed using the Kaplan–Meier method. Risk factors for the first relapse after initial RTX treatment were calculated using univariate and multivariate analysis by a Cox proportional hazard model. All data were analyzed with JMP version 11.0 (SAS institute Japan, Tokyo, Japan). Statistical significance was established at p <0.05. Adverse events (infusion reactions and late adverse events) of RTX treatment were also monitored.
Results
Characteristics of patients
The characteristics of the 81 patients are shown in Table 1. Thirty-nine patients (48 %) had a history of SRNS. All patients had a history of receiving calcineurin inhibitors (CsA or Tac) and developed SDNS under one or more IS at the time of RTX administration, resulting in severe steroid toxicity.
Results after initial RTX treatment
Steroid discontinuation after initial RTX treatment
Twelve patients (15 %) suffered from relapses before steroid discontinuation. Among them, 3 patients were able to discontinue PSL after one relapse, 1 patient discontinued it after two relapses, 5 patients discontinued it after frequent relapses and additional RTX treatments, while 3 patients could not discontinue it during the observation period because of steroid-dependent relapses in 2 patients and adrenal insufficiency in 1 patient. The other 69 patients (85 %) could discontinue PSL without relapses at a median of 66.5 days (26–409 days) after the initial RTX treatment. In total, 78 patients (97 %) eventually discontinued PSL.
IS after initial RTX treatment
All patients continued with IS and 44 patients started on MMF. Thirty-four patients who were taking mizoribine (MZR) switched to MMF. On the other hand, the number of patients using calcineurin inhibitors decreased from 72 (89 %) to 50 (62 %) at 6 months, and 43 (53 %) at 12 months. Patients on IS at 6 and 12 months after initial RTX treatment are shown in Table 2.
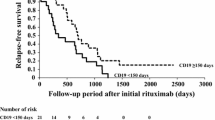
Recovery of B-cells and first relapse after initial RTX treatment
The cumulative rate of B-cell depletion and remission after initial RTX treatment is shown in Fig. 1. Three patients did not achieve B-cell depletion. B-cells recovered at a median of 160 days (range 39–311 days) after initial RTX treatment. Fifty-nine patients (73 %) suffered from relapses at a median of 309 days (0–1,201 days) after initial RTX treatment. Fifty percent relapse-free survival was 482 days. Seven patients (9 %) suffered from relapses during B-cell depletion.
Risk factors for first relapse after initial RTX treatment
Table 3 shows the risk factors for the first relapse after the initial RTX treatment by univariate and multivariate analyses using the Cox proportional hazard model. A history of SRNS and CsA use after RTX treatment was shown to be a significant risk factor in univariate analysis. However, only a history of SRNS was calculated to be a significant independent risk factor in the multivariate analysis.
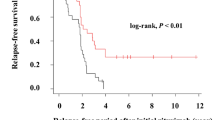
Figure 2 shows the cumulative remission rate after initial rituximab treatment stratified by the existence of a history of SRNS. Twenty-five out of 42 patients (60 %) without a history of SRNS suffered from relapses, while 34 out of 39 patients (87 %) with a history of SRNS developed relapses. The time of sustained remission in patients without a history of SRNS was significantly longer than that in patients with a history of SRNS.
Cumulative remission rate after initial rituximab (RTX) treatment stratified by a history of steroid-resistant nephrotic syndrome (SRNS) using a Kaplan–Meier survival curve. Dotted line represents patients with a history of SRNS (50 % survival, 393 days) and the continuous line represents patients without a history of SRNS (50 % survival, 615 days; p = 0.005, log-rank test)
Long-term outcome
Relapses during the observation period
Fifty-nine patients (73 %) suffered from 1–16 relapses (260 relapses in total) during the observational period. Eight patients suffered from relapses with steroid resistance. Frequencies of relapses in a year (mean ± SD) were 4.5 ± 1.9 (before RTX treatment, n = 81), 0.9 ± 1.4 (0–1 year, n = 81), 1.0 ± 1.4 (1–2 years, n = 66), 0.9 ± 1.3 (2–3 years, n = 44), 1.1 ± 1.2 (3–4 years, n = 30), 0.5 ± 1.1 (4–5 years, n = 17), 0.1 ± 0.4 (5–6 years, n = 7), and 0.0 ± 0.0 (6–7 years, n = 2). In total, the mean frequency of relapses in a year was 0.9 after RTX treatment. There were no patients with decreased renal function at the last observation.
Additional RTX treatment
Fifty-one patients (63 %) received additional RTX treatments for relapses. Seven patients received more than five additional RTX treatments.
IS at the last observation
Twelve patients (12 %) had discontinued IS by the last observation. However, 19 patients (23 %) were still using calcineurin inhibitors and 14 patients (17 %) were under two IS at the last observation.
Adverse events
Infusion reactions
In total, RTX was administered 215 times. Infusion reactions (adverse events in 24 h after the RTX infusion) were observed in 117 infusions (54 %). Table 4 shows all infusion reactions. Fifty-one patients showed more than one symptom with one infusion. Respiratory symptoms were the most frequent event. Ninety percent of infusion reactions developed within 3 h after initiation of RTX infusion. There were no serious infusion reactions and all patients were able to complete treatment.
Late adverse events
Late adverse events were observed in 15 patients (agranulocytosis, n = 9; Pneumocystis jirovecii, n = 1; herpes stomatitis, n = 1; chronic gastroenteritis, n = 1; enterocolitis and diverticulitis, n = 1; skin rash, n = 1; chronic cough and gross hematuria, n = 1). One patient suffered from two episodes of agranulocytosis. Eight of the ten episodes of agranulocytosis were accompanied by infections, but were successfully treated with antibiotics and granulocyte colony-stimulating factor. There were no life-threatening adverse events.
Discussion
Rituximab has a promising effect for preventing relapses in patients with SDNS, especially during B-cell depletion. However, disease activity is largely dependent on B-cell depletion. Two of our previous reports of case series showed that patients who continued with IS experienced longer remission than those who did not [4, 7]. This current study further confirms that finding, with remission achieved for more than 1 year with post-RTX IS.
While RTX is relatively tolerable, it can cause lethal adverse events such as progressive leukoencephalopathy (PML) [17] and interstitial pneumonia [18]. These rare but serious adverse events occur during the period of B-cell depletion. An 8-year-old girl with nephrotic syndrome developed interstitial pneumonia and expired after RTX treatment [19], while a 7-year-old boy with nephrotic syndrome developed viral myocarditis due to RTX treatment and received cardiac transplantation [20]. In our patients, although there were no lethal adverse events, late adverse events occurred in 15 cases during the B-cell depletion period. In view of this, we had to be mindful of any clinically serious delayed-onset complications that could potentially cause serious bacterial infections, such as sepsis. Monitoring complete blood counts and CD19+ B-cell counts at least once a month until B-cell recovery after RTX use is helpful for the early detection of agranulocytosis and the prevention of serious infections [21]. Before this study, we also treated a 3-year-old boy with Pneumocystis jirovecii [22]. As such, the period of B-cell depletion should be shortened whenever possible. We believe that our strategy of post-RTX IS is an effective option for the prevention of relapse without a prolonged period of B-cell depletion.
Our results show that a history of SRNS is an independent risk factor for relapse after RTX treatment. When patients with SRNS achieve complete remission, they often develop sensitivity to steroids and refractory SDNS under IS. As patients with a history of SRNS might be included as a severe form of SDNS, it could be difficult to control disease activity and the potential resistance against our combination strategy of RTX with IS. We believe that additional treatment with a single dose of RTX at B-cell recovery for prevention from relapse might be a suitable option for patients with a history of SRNS, although further investigation is necessary.
It remains unclear whether RTX itself weakens disease activity even after B-cell recovery. In our study, the frequency of relapses in a year noticeably decreased compared with pre-RTX, and this lower frequency was maintained for several years with continued IS and repeated treatment of RTX. Hence, we speculate that RTX might weaken disease activity and improve the efficacy of IS, although the exact mechanism is unknown. Notably, 89 % of the patients were under the treatment of calcineurin inhibitors at first RTX administration, but only 23 % were still using them at the last observation. We believe that the calcineurin-sparing effect of RTX can be useful for SDNS patients, as most of them are likely to develop CsA dependence, potentially leading to CsA nephrotoxicity [1]. We think that MMF may be a better option than calcineurin inhibitors, as it allows prolonged use without the side effect of nephrotoxicity [23].
There are several limitations to our study. First, it is a retrospective observational study. Nonetheless, the number of patients is to our knowledge the largest presented in the literature to date. Second, the observation period is different in each patient. However, we were able to follow them for at least a year. Third, IS treatment and discontinuation of steroids after RTX were different for each patient as the protocol was not restricted. Fourth, although a reduction in the number of relapses was achieved by our strategy, its clinical effectiveness was not precisely proven, as more than half of patients received additional RTX treatments. To verify the effectiveness of our strategy, we are planning a multicenter randomized controlled study to compare the effect of RTX alone with RTX followed by MMF.
In conclusion, RTX treatment followed by IS is a promising and effective option for patients with SDNS. Almost all the patients were able to discontinue steroid treatment and some achieved long-term remission. Moreover, as the number of patients using calcineurin inhibitors was dramatically decreased at the last observation, a calcineurin-sparing effect of RTX can also be expected. Notably, a history of SRNS is a risk factor for early relapse after RTX treatment. Further investigation and prospective study are needed to establish the strategy of RTX treatment combined with IS for SDNS.
References
Iijima K, Hamahira K, Tanaka R, Kobayashi A, Nozu K, Nakamura H, Yoshikawa N (2002) Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int 61:1801–1805
Latta K, von Schnakenburg C, Ehrich JH (2001) A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 16:271–282
Kamei K, Ito S, Nozu K, Fujinaga S, Nakayama M, Sako M, Saito M, Yoneko M, Iijima K (2009) Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol 24:1321–1328
Fujinaga S, Hirano D, Nishizaki N, Kamei K, Ito S, Ohtomo Y, Shimizu T, Kaneko K (2010) Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol 25:539–544
Gulati A, Sinha A, Jordan SC, Hari P, Dinda AK, Sharma S, Srivastava RN, Moudgil A, Bagga A (2010) Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 5:2207–2212
Sellier-Leclerc AL, Macher MA, Loirat C, Guérin V, Watier H, Peuchmaur M, Baudouin V, Deschênes G (2010) Rituximab efficiency in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 25:1109–1115
Ito S, Kamei K, Ogura M, Sato M, Fujimaru T, Ishikawa T, Udagawa T, Iijima K (2011) Maintenance therapy with mycophenolate mofetil after rituximab in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:1823–1828
Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM (2011) Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 6:1308–1315
Sinha A, Bagga A, Gulati A, Hari P (2012) Short-term efficacy of rituximab versus tacrolimus in steroid-dependent nephrotic syndrome. Pediatr Nephrol 27:235–241
Ito S, Kamei K, Ogura M, Udagawa T, Fujinaga S, Saito M, Sako M, Iijima K (2013) Survey of rituximab treatment for childhood-onset refractory nephrotic syndrome. Pediatr Nephrol 28:257–264
Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G, Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group (2014) Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25:850–863
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y, Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group (2014) Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384:1273–1281
Kemper MJ, Gellermann J, Habbig S, Krmar RT, Dittrich K, Jungraithmayr T, Pape L, Patzer L, Billing H, Weber L, Pohl M, Rosenthal K, Rosahl A, Mueller-Wiefel DE, Dötsch J (2012) Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 27:1910–1915
Sellier-Leclerc AL, Baudouin V, Kwon T, Macher MA, Guérin V, Lapillonne H, Deschênes G, Ulinski T (2012) Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood—follow-up after CD19 recovery. Nephrol Dial Transplant 27:1083–1089
Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, Bodria M, Caridi G, Wei C, Belingheri M, Ghio L, Merscher-Gomez S, Edefonti A, Pasini A, Montini G, Murtas C, Wang X, Muruve D, Vaglio A, Martorana D, Pani A, Scolari F, Reiser J, Ghiggeri GM (2013) Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84:1025–1033
Tellier S, Brochard K, Garnier A, Bandin F, Llanas B, Guigonis V, Cailliez M, Pietrement C, Dunand O, Nathanson S, Bertholet-Thomas A, Ichay L, Decramer S (2013) Long-term outcome of children treated with rituximab for idiopathic nephrotic syndrome. Pediatr Nephrol 28:911–918
Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Winter JN, Furman RR, Vose JM, Zelenetz AD, Mamtani R, Raisch DW, Dorshimer GW, Rosen ST, Muro K, Gottardi-Littell NR, Talley RL, Sartor O, Green D, Major EO, Bennett CL (2009) Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113:4834–4840
Bitzan M, Anselmo M, Carpineta L (2009) Rituximab (B-cell depleting antibody) associated lung injury (RALI): a pediatric case and systematic review of the literature. Pediatr Pulmonol 44:922–934
Chaumais MC, Garnier A, Chalard F, Peuchmaur M, Dauger S, Jacqz-Agrain E, Deschênes G (2009) Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol 24:1753–1755
Sellier-Leclerc AL, Belli E, Guérin V, Dorfmüller P, Deschênes G (2013) Fulminant viral myocarditis after rituximab therapy in pediatric nephrotic syndrome. Pediatr Nephrol 28:1875–1879
Kamei K, Takahashi M, Fuyama M, Saida K, Machida H, Sato M, Ogura M, S (2015) Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature. Nephrol Dial Transplant 30:91–96
Sato M, Ito S, Ogura M, Kamei K, Miyairi I, Miyata I, Higuchi M, Matsuoka K (2013) Atypical Pneumocystis jiroveci pneumonia with multiple nodular granulomas after rituximab for refractory nephrotic syndrome. Pediatr Nephrol 28:145–149
Fujinaga S, Sakuraya K, Yamada A, Urushihara Y, Ohtomo Y, Shimizu T (2015) Positive role of rituximab in switching from cyclosporine to mycophenolate mofetil for children with high-dose steroid-dependent nephrotic syndrome. Pediatr Nephrol 30:687–691
Acknowledgements
The authors thank Drs K. Tanaka (Aomori), K. Tsuruga (Aomori), T. Echizenya (Iwate), D. Ogino (Yamagata), T. Watanabe (Gumma), Y. Owada (Tochigi), R. Hiramoto (Chiba), H. Eguchi (Chiba), M. Hisano (Chiba), Z. Kiuchi (Tokyo), K. Saida (Tokyo), H. Hataya (Tokyo), K Ishikura (Tokyo), E. Tanaka (Tokyo), M. Takahashi (Tokyo), E. Kikuchi (Tokyo), T. Udagawa (Tokyo), M. Okada (Tokyo), T. Fujimaru (Tokyo), M. Fuyama (Kanagawa), K. Honda (Kanagawa), H. Machida (Kanagawa), A. Inaba (Kanagawa), A. Ueda (Kanagawa), S. Noda (Nagano), T. Ishikawa (Nara), H. Kaito (Hyogo), M. Mizutani (Yamaguchi), M. Fujieda (Kochi), M. Ishihara (Kochi), H. Nakazato (Kumamoto), H. Nagasako (Kagoshima), A. Miyazono (Kagoshima), M. Yoshishige (Kagoshima), H. Yoshimura (Okinawa) for their contributions to this study. They would also like to thank Dr J. Tang from the Department of Education for Clinical Research, National Center for Child Health and Development, for proofreading and editing the manuscript.
Conflict of interest statement
None declared.
Ethical disclosure
The study protocol was based on the Declaration of Helsinki and approval of the off-label use of RTX was obtained from the ethics committee of our center (#645). All patients’ parents gave written informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamei, K., Ogura, M., Sato, M. et al. Risk factors for relapse and long-term outcome in steroid-dependent nephrotic syndrome treated with rituximab. Pediatr Nephrol 31, 89–95 (2016). https://doi.org/10.1007/s00467-015-3197-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3197-0