Abstract
Strategies to facilitate repair or generate new nephrons are exciting prospects for acute and chronic human renal disease. Repair of kidney injury involves not just local mechanisms but also mobilisation of progenitor/stem cells from intrarenal niches, including papillary, tubular and glomerular locations. Diverse markers characterise these unique cells, often including CD24 and CD133. Extrarenal stem cells may also contribute to repair, with proposed roles in secreting growth factors, transfer of microvesicles and exosomes and immune modulation. Creating new nephrons from stem cells is beginning to look feasible in mice in which kidneys can be dissociated into single cells and will then generate mature renal structures when recombined. The next step is to identify the correct human markers for progenitor cells from the fetus or mature kidney with similar potential to form new kidneys. Intriguingly, development can continue in vivo: whole foetal kidneys and recombined organs engraft, develop a blood supply and grow when xenotransplanted, and there are new advances in decellularised scaffolds to promote differentiation. This is an exciting time for human kidney repair and regeneration. Many of the approaches and techniques are in their infancy and based on animal rather than human work, but there is a rapid pace of discovery, and we predict that therapies based on advances in this field will come into clinical practice in the next decade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current treatments for end-stage kidney disease (chronic kidney disease stage 5; CKD5) are limited to dialysis or, if the patient is fortunate, renal transplantation. Quality of life (QOL) of patients with CKD5 is compromised at whatever age, but it is particularly hard for children, who should be growing and developing without fluid and dietary restrictions on the one hand and medication and time-consuming treatments on the other. There are 870 children in the UK under the age of 18 years with CKD5 [1]. Many have congenital kidney or lower urinary tract malformations, such as renal dysplasia and reflux nephropathy (32.6 %) or obstructive uropathy (17.3 %), and there has been a 50 % rise in these in children <2 years in the last decade. Adult CKD5 numbers are much higher: around 8,000 individuals are on the renal waiting list for a transplant in the UK, but only around a quarter receive one each year. There were 110,000 adults on US transplant registries in 2011, but only 13,430 transplants were performed. Renal costs now account for 3 % of the annual budget of the UK National Health Service, and CKD costs were estimated at US $41 billion for Medicare patients alone in the USA [2]. There is an urgent need for better treatment to prevent or improve CKD in children and adults. One long-term hope, explored in this review, is that it may become possible to deliver progenitor cell therapies that either repair or help regenerate existing structures or generate completely new nephrons. It is highly unlikely that we will ever be able to generate perfect kidneys with hundreds of thousands of functioning nephrons, but any therapy that safely delays progression to CKD5 would be welcome. The challenge will be to find the correct cells to use and timing the therapy for maximal effect.
Repair/regeneration of existing structures
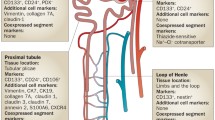
Acute and chronic kidney injury have different aetiologies and affect different cells but can perhaps be regarded as a continuous spectrum of damage, as repeated acute insults often progress to chronic disease. Acute injury often highlights the proximal tubule, which is particularly metabolically active and may reabsorb/concentrate toxins or the glomerulus, which is not only exposed to high vascular shear forces but is also a common site for immune-mediated pathology; in contrast, chronic disease tends to be more widespread (albeit often patchy), with interstitial fibrosis, tubular atrophy and microvascular loss [3, 4]. Cell turnover is low in the normal mature kidney but dysregulated in injury/disease [5], and the key to maximising repair/recovery and preventing CKD progression must be to target the correct cells to the correct place within the correct time. Most renal injury is repaired by intrarenal cells, either local neighbouring cells of the same type or, more controversially, stem-like cells [6–8]. Organs such as skin and gut definitely contain stem cells that contribute to tissue maintenance and repair, but it is only over the last few years that similar cell types have been recorded in the kidney [6, 7, 9–11]. Totipotent cells parallel true embryonic stem cells in that they are: (i) undifferentiated, (ii) able to self-renew repeatedly and (iii) can give rise to cell lineages from all three germ layers. Kidney stem/progenitor cells have been identified that can specifically regenerate renal tissues by functional and expression studies documenting a low intrinsic proliferative rate (until stimulated) with label retention, extrusion of fluorescent dyes and high aldehyde dehydrogenase (ALDH) activity; plus expression of cell-surface markers, such as CD24, CD133 and others (see Table 1 and [12]). Three renal progenitor niches have been reported: renal papilla, tubules and parietal layer of Bowman’s capsule in the glomerulus (Fig. 1).
Kidney development and sites of intrarenal stem cells. a–c Nephrogenesis. a Outgrowth of the ureteric bud (ub) from the Wolffian duct (wd) into the metanephric mesenchyme (mm) at 5 weeks of human gestation. b Branching of the bud (bb) with each ampullary tip stimulating the mesenchyme to condense around it (cm) and begin to undergo mesenchymal–epithelial conversion; 6 weeks gestation. c Further branching and stages of nephron formation from renal vesicle (v), through comma shapes (c) to maturing glomeruli. d, e Labeled for potential sites of stem/progenitor cells, such as the tips of the ureteric bud branches and early condensed mesenchyme (d) and the papillary region, parietal epithelial glomerular cells and diffuse tubular cells particularly in proximal tubules (e)
Oliver and colleagues reported label-retaining cells in the mouse papilla [9]: they identified slowly cycling cells using a pulse of bromodeoxyuridine (BrdU) in mice and rat pups, followed by a prolonged 2-month chase; the only area with numerous labelled cells was the papilla. These cells entered the cell cycle and moved out of the papilla following renal injury and formed spheres in vitro as well as potential differentiation towards other lineages [9]. The niche was subsequently refined to the upper papilla [13], and a different group demonstrated similar cells in the mouse papilla. The authors found expression of the developmental marker Pax2, persistent self-renewal and potential for differentiation into diverse renal lineages [14]. Comparable cells reside in human kidneys: Ward el al. demonstrated double nestin and CD133/1+ cells in papillae that displayed integration into tubular structures in 3D culture and after transplant into developing mouse kidneys [15].
Label-retaining cells can also be found in proximal tubules [16]. These cells proliferate after renal injury and divide asymmetrically, generating different populations, some of which remained undifferentiated (progenitors for the future perhaps?), whereas others generated epithelia. Multipotent progenitor cells have been isolated from rat kidneys with spindle-shaped morphology, prolonged self-renewal for >200 passages and expression of vimentin, CD90 (thy1.1), Pax-2 and Oct4, but not more differentiated markers such as cytokeratin or major histocompatibility complex (MHC) class I or II [10]. These are able to differentiate into renal tubules after subcapsular or intra-arterial injection. CD133+ cells can be found in the tubular fraction of normal adult kidneys, coexpressing PAX2 [11]. These can also contribute to different lineages, including renal tubule epithelia and endothelia. A recent study has further highlighted a triple CD24, CD133 and vimentin-positive subpopulation of cells scattered throughout the proximal tubule; this population expanded in rat unilateral ureteral obstruction, reiterating possible roles in tubular regeneration [17]. Similarly, triple CD24, CD133 and CD106+ cells from human cortical tubules and glomeruli have the potential to engraft in to regenerating tubules in experimental renal injury [18]. Other candidate markers for human progenitor cells are summarised in Table 1, and include ALDH [19] and toll-like receptor 2 (TLR2) [20, 21].
Potential podocyte and proximal nephron progenitors localise to parietal epithelial cells of Bowman’s capsule: a subset of parietal epithelial cells express CD133 and CD24; moreover, they exhibit clonal self-renewal and generate lineages with features of both proximal and distal tubule epithelia [22]. CD133+CD24+ cells are in the renal vesicles and S-shaped bodies in developing human kidneys but were later restricted to the urinary pole of Bowman’s capsule [23]. Other markers distinguish subsets within these double-positive cells, with a key factor appearing to be podocalyxin: CD24+CD133+podocalyxin-negative cells localise close to the urinary pole, whereas triple-positive cells concentrate towards the vascular pole; only the former have significant potential to generate tubular cells or podocytes or improve adriamycin-induced podocyte damage [24, 25].
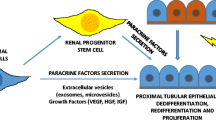
Despite the evidence that the bulk of cells involved in the normal processes invoked to repair renal injury are intrarenal [6, 7], there is emerging data that exogenous stem/progenitor cells might add to future therapeutic options: bone marrow cells migrate to the kidney and participate in normal tubular epithelial cell turnover and repair after acute kidney injury [26]; mesenchymal stem cells improve renal recovery in models of injury [27]; endothelial progenitor cells may also contribute to repair [28]. Amniotic-fluid-derived cells may also be therapeutic in experimental renal injury [29, 30], whereas induced pluripotent stem (IPS) cells can differentiate towards renal lineages in vitro and in vivo [31]. Progenitor/stem cells not only replace damaged kidney cells; they secrete growth factors, cytokines and chemokines; release microvesicles or exosomes that can transduce regenerative signals and even mitochondria into target cells [32] and modulate the immune response; alter the balance from apoptosis to proliferation of target cells and promote angiogenesis [32]. Infused mesenchymal stem cells are removed from the body within 24 h, so positive effects must initiate early in injury and be renal targeted for maximal effects [33].
Generation of nephrons de novo
There may be too few nephrons to repair in bilateral congenital malformations or towards the end stage; hence, we need a different strategy to avoid CKD5, namely, generating new nephrons. The ideal plan would be to grow kidneys from patient-derived cells, such as renal progenitor/stem cells isolated after kidney biopsy, or reprogrammed IPS or amniotic-fluid-derived cells. It is important to understand the processes and molecules involved in kidney development in order to produce appropriate cells to generate nephrons. The kidney is derived from intermediate mesoderm, and the key interactions needed to build the organ are repeated interactions between two cell types: epithelia in the ureteric bud, and mesenchyme in the metanephric blastema. In humans, the permanent kidney (the metanephros) initiates at 5 weeks of gestation, when the ureteric bud invades the metanephric mesenchyme (Fig. 1). The ureteric bud branches multiple times to generate the collecting duct system, whereas induced mesenchyme around the tips of the ureteric bud condenses into aggregates that epithelialise and then elongate to form the nephron from glomerulus to distal tubule. The first human glomeruli are observed at about 9 weeks of gestation. Proliferation, branching and differentiation cycles continue until 32–36 weeks gestation, when nephrogenesis finishes (i.e. before birth in humans). Numerous groups have isolated ureteric buds/lines and demonstrate branching and collecting-duct development [34, 35], but most research has focussed on the mesenchyme, particularly the inducers needed to force epithelial conversion.
It has been known for many years that the spinal cord induces epithelial conversion of metanephric mesenchyme, as do other cultures that stimulate Wnt signalling [36]. However, little was known about factors that promote conversion until two groups identified leukaemia inhibitory factor (LIF) as the critical component in this process [37, 38]. These studies demonstrated tubule formation in lumps of isolated rat mesenchymes, and our group set out to investigate whether similar conditions worked for human progenitor cells [39] (Fig. 2). We explanted sections of 8- to 12-week human metanephroi, anonymously collected with full consent and ethical approval, and allowed cells to grow out. Colonies were initially of mixed epithelial and mesenchymal phenotype, but the latter grew much faster and populations became entirely mesenchymal in appearance and gene expression by the second passage, remaining viable for >20 passages (the furthest we cultured). The cells expressed classical mesenchymal markers (Fig. 2) but did not epithelialise with the combination of LIF/fibroblast growth factor 2 (FGF2)/transforming growth factor alpha (TGFα) sufficient to induce rat epithelial transformation, although we did observe a number of novel gene-expression changes in factors such as matrix metalloproteinase 1, stanniocalcin and the neural stem cell marker Nestin [39]. Culturing the cells longer in our standard serum-free media led to clump formation and detachment by 7 days, which was reversed by further culture in normal medium, suggesting that this effect was not a sign of toxicity, and even restoration of 5 % serum did not help differentiation (Fig. 2 and unpublished data). These findings raise intriguing questions about potential differences between human and rodent kidney development, which must be borne in mind with much of the murine work described below.
Isolation and characterisation of potential progenitor cells from human foetal kidneys. a Explant slices of a 10-week-gestation human kidney showing outgrowth of cells. b, c Mesenchymal cell lines express characteristic markers of induced mesenchyme, such as WT1 and PAX2; produce mesenchymal-derived growth factors, such as hepatocyte growth factor (HGF) and glial-cell-line-derived growth factor (GDNF); and lack epithelial markers, such as E-cadherin (E-CAD), rearranged during transfection (RET) or wingless-related MMTV integration site 11 (WNT11). d–f) Human cells do not respond to leukaemia inhibitory factor (LIF) by epithelialising, unlike rat mesenchymes [37, 38]: d Classic mesenchymal phenotype in control cultures without LIF. e Cells form abnormal clumps after 7 days of LIF in serum-free culture medium; these fall off the plate but regrow when LIF is removed and serum reintroduced. f No morphological effect of LIF after 7 days of culture in normal medium that includes 5 % serum
A longstanding question concerns which markers delineate the mesenchyme that is committed to forming nephrons versus that destined to become stroma. The latter lineage seems clearly marked by expression of BF2/Foxd1 [40], but a series of markers have been linked to nephron precursors at early stages of commitment, including Cited1, Osr1, Sall1 and Six2 [41, 42]. In an ideal therapy, one might isolate human cells expressing these markers, combine them with potential ureteric bud epithelia and await kidney development, but these are transcription factors rather than cell-surface markers, which makes specific isolation difficult. Therefore, different markers have been sought, particularly by Dekel and his group [43–45]: suggested key markers are NCAM, EpCAM and FZD7, whereas CD24 and CD133 are seen in more differentiated cells. A recent paper suggested that mouse embryonic stem cells expressing kidney-specific protein (KSP) have a similar molecular profile to kidney progenitors and generate tubular structures in 3D culture conditions [46]; selection via KSP may be useful for IPS cells, also.
Isolating appropriate precursors is only the first step; other questions are: (i) Can the progenitors generate nephrons/a whole kidney de novo? (ii) Will they develop and work in vivo? (iii) Can we improve structure using a preformed renal scaffold? Generating a new kidney from individual cells seemed fantastical only 5 years ago, but a number of groups have now reported this based on original work from the laboratory of Jamie Davies in Edinburgh: Unbekandt dissociated early foetal mouse kidneys into single-cell suspension and then centrifuged them into a pellet that was then cultured; too many cells were lost for successful nephrogenesis until a new step was introduced: that of blocking apoptosis using an inhibitor of Rho kinase (ROCK) during the first 24 h of culture. Typical renal structures were then formed, including nephrons and collecting ducts [47]. This simple recombination’ experiment allows the renal developmental potential of any cells to be assessed without requiring exogenous tissues or inducers, and it has been refined to promote better ureteric tree and loop of Henle development [48]. In recent work in our laboratory, we used the same strategy for human foetal kidney cells; preliminary results suggest that primary cells from early foetal stages have the potential to generate tubular and glomerular structures following recombination, but the supply of such tissues is rare, so we are now attempting to replicate and refine this process using established cell lines (unpublished data).
There are limits to kidney growth in vitro because of the lack of blood supply and reliance on diffusion for nutrient and oxygen supply. Hence, the next test of potential is whether the neo-organs will develop in vivo. Xenotransplantation of whole metanephric kidneys has been reported for rats, which showed kidney growth, vascularisation by host vessels and some degree of excretory function [49]; even early human foetal kidneys will develop in experimental hosts [50]. Xinaris recently extended this experiment with the recombination kidney technique [51]: neo-organs were cultured for 1 or 5 days, then implanted through a catheter beneath the renal capsule of unilaterally nephrectomised athymic rats and left to grow for 3 weeks; only organoids comprising large numbers of cells survived, and these only contained rudimentary glomerular-like structures initially; in this case, local and systemic vascular endothelial growth factor (VEGF) rescued nephrogenesis, generating a decent host-derived blood supply and maturing glomeruli. Neither of these experiments documented long-term growth of the neo-organs, but both indicated vascularisation, which is a prerequisite for onwards development and may be an additional area to be considered in future therapies for CKD5 [52].
Finally, might it not be easier to start with a partially built kidney rather than developing the entire structure from scratch? This is the concept used in other, less complex, organs, such as the trachea [53], but will it work for the kidney, which has complex 3D anatomy and cellular specialisation? Initial reports are promising using a strategy in which normal kidneys are decellularised using prolonged flushing with detergent to clear cells whilst retaining structure- and segment-specific matrix proteins, such as the collagens, laminin, fibronectin and glycosaminoglycans. Ross and colleagues decellularised rat kidneys and then perfused them with mouse embryonic stem cells either through the artery or ureter; precursor cells populated and proliferated, expressing some immunohistochemical markers for differentiation [54]. Other scaffolds have derived from rhesus monkey and surplus human kidneys. The most compelling results reported thus far, however, come from Song and colleagues, who implanted a rat scaffold populated with human umbilical venous endothelial cells (HUVECs) through the renal artery and rat neonatal kidney cells (NKCs) through the ureter [55]. The neo-organ picked up a vascular supply from the host and produced urine, albeit of low quantity and quality. Taking the scaffold concept one step further, it may even be possible to print a kidney scaffold and populate it with segment-specific cells [56].
Conclusion
This is an exciting time for kidney repair and regeneration. Almost every month there are new markers identified for kidney stem/progenitor cells. Our ability to manipulate cell phenotype and fate is advancing rapidly, and progress in neo-organ recombination and biomaterials is breathtaking. These advances are important because there is an urgent need for new therapies in both congenital and acquired kidney diseases, but we must inject an element of caution, as much of the data comes from animal work, and human studies are clearly more complex and difficult. Nevertheless, we predict a bright future for targeted nephron repair and neonephrogenesis; it is our hope that therapies based on these principles will be developed for clinical practice within the next decade.
References
Caskey F, Dawnay A, Farrington K, Feest T, Garty D, Inward C, Tomson CRV (2011) UK Renal Registry 2010. 13th Annual Report of the Renal Association. Nephron Clin Pract 119(suppl 2)
U.S. Renal Data System, USRDS 2013 Annual Data Report (2013) Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.Available at http://www.usrds.org/adr.aspx
Sharfuddin AA, Molitoris BA (2011) Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7:189–200
Chawla LS, Kimmel PL (2012) Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82:516–524
Winyard PJD, Nauta J, Lirenman DS, Hardman P, Sams VR, Risdon RA, Woolf AS (1996) Deregulation of cell survival in cystic and dysplastic renal development. Kidney Int 49:135–146
Lin F, Moran A, Igarashi P (2005) Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115:1756–1764
Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2:284–291
Togel FE, Westenfelder C (2012) Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis 60:1012–1022
Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q (2004) The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114:795–804
Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME (2006) Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol 17:3028–3040
Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G (2005) Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166:545–555
McCampbell KK, Wingert RA (2012) Renal stem cells: fact or science fiction? Biochem J 444:153–168
Oliver JA, Klinakis A, Cheema FH, Friedlander J, Sampogna RV, Martens TP, Liu C, Efstratiadis A, Al-Awqati Q (2009) Proliferation and migration of label-retaining cells of the kidney papilla. J Am Soc Nephrol 20:2315–2327
Fuente MC, Ranghini E, Bruno S, Bussolati B, Camussi G, Wilm B, Edgar D, Kenny SE, Murray P (2012) Differentiation of podocyte and proximal tubule-like cells from a mouse kidney-derived stem cell line. Stem Cells Dev 21:296–307
Ward HH, Romero E, Welford A, Pickett G, Bacallao R, Gattone VH, Ness SA, Wandinger-Ness A, Roitbak T (2011) Adult human CD133/1(+) kidney cells isolated from papilla integrate into developing kidney tubules. Biochim Biophys Acta 1812:1344–1357
Maeshima A, Yamashita S, Nojima Y (2003) Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 14:3138–3146
Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, Moeller MJ (2013) Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229:645–659
Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P (2012) Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30:1714–1725
Lindgren D, Bostrom AK, Nilsson K, Hansson J, Sjolund J, Moller C, Jirstrom K, Nilsson E, Landberg G, Axelson H, Johansson ME (2011) Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol 178:828–837
Sallustio F, De Benedictis L, Castellano G, Zaza G, Loverre A, Costantino V, Grandaliano G, Schena FP (2010) TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J 24:514–525
Sallustio F, Costantino V, Cox SN, Loverre A, Divella C, Rizzi M, Schena FP (2013) Human renal stem/progenitor cells repair tubular epithelial cell injury through TLR2-driven inhibin-A and microvesicle-shuttled decorin. Kidney Int 83:392–403
Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P (2006) Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17:2443–2456
Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P (2007) Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18:3128–3138
Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P (2009) Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20:322–332
Bruno S, Bussolati B, Grange C, Collino F, di Cantogno LV, Herrera MB, Biancone L, Tetta C, Segoloni G, Camussi G (2009) Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Stem Cells Dev 18:867–880
Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA (2001) Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195:229–235
Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C (2005) Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 68:1613–1617
Ratliff BB, Goligorsky MS (2013) Delivery of EPC embedded in HA-hydrogels for treatment of acute kidney injury. Biomatter 3:e23284
De CP, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106
Rota C, Imberti B, Pozzobon M, Piccoli M, De CP, Atala A, Gagliardini E, Xinaris C, Benedetti V, Fabricio AS, Squarcina E, Abbate M, Benigni A, Remuzzi G, Morigi M (2012) Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev 21:1911–1923
Mae S, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, Arai S, Sato-Otubo A, Toyoda T, Takahashi K, Nakayama N, Cowan CA, Aoi T, Ogawa S, McMahon AP, Yamanaka S, Osafune K (2013) Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. doi:10.1038/ncomms2378
Gnecchi M, Zhang Z, Ni A, Dzau VJ (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circes 103:1204–1219
Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ (2012) Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 3:297. doi:10.3389/fimmu.2012.00297
Steer DL, Shah MM, Bush KT, Stuart RO, Sampogna RV, Meyer TN, Schwesinger C, Bai X, Esko JD, Nigam SK (2004) Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol 272:310–327
Zhang X, Bush KT, Nigam SK (2012) In vitro culture of embryonic kidney rudiments and isolated ureteric buds. Methods Mol Biol 886:13–21, d
Herzlinger D, Qiao J, Cohen D, Ramakrishna N, Brown AM (1994) Induction of kidney epithelial morphogenesis by cells expressing Wnt-1. Dev Biol 166:815–818
Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA (1999) Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 99:377–386
Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO (2001) TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development 128:1045–1057
Price KL, Long DA, Jina N, Liapis H, Hubank M, Woolf AS, Winyard PJ (2007) Microarray interrogation of human metanephric mesenchymal cells highlights potentially important molecules in vivo. Physiol Genomics 28:193–202
Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E (1996) Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev 10:1467–1478
Kiefer SM, Robbins L, Stumpff KM, Lin C, Ma L, Rauchman M (2010) Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development 137:3099–3106
Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G (2006) Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25:5214–5228
Metsuyanim S, Harari-Steinberg O, Buzhor E, Omer D, Pode-Shakked N, Ben-Hur H, Halperin R, Schneider D, Dekel B (2009) Expression of stem cell markers in the human fetal kidney. PLoS One 4:e6709
Harari-Steinberg O, Metsuyanim S, Omer D, Gnatek Y, Gershon R, Pri-Chen S, Ozdemir DD, Lerenthal Y, Noiman T, Ben-Hur H, Vaknin Z, Schneider DF, Aronow BJ, Goldstein RS, Hohenstein P, Dekel B (2013) Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med 5:1556–1568
Buzhor E, Omer D, Harari-Steinberg O, Dotan Z, Vax E, Pri-Chen S, Metsuyanim S, Pleniceanu O, Goldstein RS, Dekel B (2013) Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. Am J Pathol. doi:10.1016/j.ajpath.2013.07.034
Morizane R, Monkawa T, Fujii S, Yamaguchi S, Homma K, Matsuzaki Y, Okano H, Itoh H (2013) Kidney specific protein-positive cells derived from embryonic stem cells reproduce tubular structures in vitro and differentiate into renal tubular cells. PLoS One 8:e64843
Unbekandt M, Davies JA (2010) Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int 77:407–416
Chang CH, Davies JA (2012) An improved method of renal tissue engineering, by combining renal dissociation and reaggregation with a low-volume culture technique, results in development of engineered kidneys complete with loops of henle. Nephron Exp Nephrol 121:e79–e85
Hammerman MR (2005) Cellular transplantation of nephrons. Kidney Int 67:1677–1679
Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, Rechavi G, Friedman N, Kaminski N, Passwell JH, Reisner Y (2003) Human and porcine early kidney precursors as a new source for transplantation. Nat Med 9:53–60
Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, Conti S, Unbekandt M, Davies JA, Morigi M, Benigni A, Remuzzi G (2012) In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol 23:1857–1868
Long DA, Norman JT, Fine LG (2012) Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol 8:244–250
Fishman JM, De CP, Elliott MJ, Atala A, Birchall MA, Macchiarini P (2011) Airway tissue engineering. Expert Opin Biol Ther 11:1623–1635
Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD (2009) Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol 20:2338–2347
Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC (2013) Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19:646–651
Partridge R, Conlisk N, Davies JA (2012) In-lab three-dimensional printing: an inexpensive tool for experimentation and visualization for the field of organogenesis. Organogenesis 8:22–27
Sources of support
This work was supported by Kids Kidney Research, Kidney Research UK and the National Institute for Health Research Biomedical Research Centre Funding Scheme. Material supplied by the MRC/Wellcome funded Human Developmental Biology Resource is gratefully acknowledged. This report is independently produced and the views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winyard, P.J.D., Price, K.L. Experimental renal progenitor cells: Repairing and recreating kidneys?. Pediatr Nephrol 29, 665–672 (2014). https://doi.org/10.1007/s00467-013-2667-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2667-5