Abstract
The kidney is a complex organ with more than 20 types of specialized cells that play an important role in maintaining the body’s homeostasis. The epithelial tubular cell is formed during embryonic development and has little proliferative capacity under physiological conditions, but after acute injury the kidney does have regenerative capacity. However, after repetitive or severe lesions, it may undergo a maladaptation process that predisposes it to chronic kidney injury. Regenerative medicine includes various repair and regeneration techniques, and these have gained increasing attention in the scientific literature. In the future, not only will these techniques contribute to the repair and regeneration of the human kidney, but probably also to the construction of an entire organ. New mechanisms studied for kidney regeneration and repair include circulating stem cells as mesenchymal stromal/stem cells and their paracrine mechanisms of action; renal progenitor stem cells; the leading role of tubular epithelial cells in the tubular repair process; the study of zebrafish larvae to understand the process of nephron development, kidney scaffold and its repopulation; and, finally, the development of organoids. This review elucidates where we are in terms of current scientific knowledge regarding these mechanisms and the promises of future scientific perspectives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successive episodes of renal injury or acute severe kidney injury may increase the risk for the development of chronic kidney disease (CKD) and the need for renal replacement therapy (RRT). With this in mind, regenerative medicine is a promising field in the development of therapies for kidney disease. Regenerative medicine is an interdisciplinary area that can extend from restoring damaged tissue to developing an entire organ. It is a promising area of kidney disease treatment and kidney replacement therapy and involves various strategies for the regeneration and/or repair of an injured area, as well as the creation of an entire kidney for organ transplantation, as listed bellow.
-
1.
Repair and regeneration of the kidney
-
a)
Use of circulating stem cells: mesenchymal stem/stromal cells (MSCs)
-
b)
Use of MSC paracrine factors: growth factors, cytokines, extracellular vesicles
-
c)
Study of the interaction of MSCs with resident stem/progenitor cells: kidney progenitor stem cells (PSCs)
-
d)
Study of tubular epithelial cell participation in repair/regeneration
-
a)
-
2.
Rebuilding the entire organ for transplant
-
a)
Zebrafish kidney development model to conduct human kidney regeneration
-
b)
Tissue bioengineering: repopulation of kidney scaffolds and development of organoids
-
a)
This field is growing rapidly, which makes it increasingly difficult to follow the different strategies and techniques applied in regeneration. Therefore, rather than depicting a specific method, in this review we focus on analyzing the main strategies studied in kidney regenerative medicine to date and discussing what remains to be done in terms of regenerative medicine for kidney diseases.
Kidney repair and regeneration
Circulating stem cells: mesenchymal stromal cells
Therapy with stem cells is the most advanced regenerative therapy strategy to date and the best investigated. Nevertheless, some issues must be raised, as there are differences among the types of stem cells available for regenerative therapy. Embryonic and pluripotent induced stem cells are those with the most differentiation potency and they also seem to have the greatest teratogenic capacity [1, 2]. Umbilical cord or Wharton’s jelly stromal cells appear to have the highest proliferative capacity, and they are not altered due to aging or environmental stresses [3, 4]. However, bone marrow MSCs are the most studied cell type at the present time and probably the safest for use in clinical trials [5].
MSCs are cells which have the inherent ability to self-renew and differentiate into cells of mesenchymal lineage. They are obtained from the bone marrow, among other cells. The minimum requirements for the characterization of MSCs are the expression of CD (cluster of differentiation) 90, CD73, and CD105 surface markers and the absence of expression of CD45, CD34, CD11b, CD19, and HLA-DR markers. Other criteria are the capacity to adhere to a plastic surface under culture conditions and to differentiate into osteoblasts, adipocytes, and chondrocytes [6, 7].
The number of publications on MSCs increase yearly. Several clinical projects have already been carried out, including those regarding the use of MSCs in the area of kidney disease, as such studies are promising for future clinical applications [5]. MSCs have inherent homing and persistence abilities at the site of injury. It is generally assumed that these stem cells follow the same steps as those described for leukocyte homing, including contact with the endothelium by tethering and rolling, cellular activation mediated by membrane receptors, and cellular arrest and transmigration [8]. The expression of the membrane glycoprotein CD44 is important in MSCs at all of these steps. Chemokine receptors involved in MSC activation are the CXCR4 receptor, activated by SDF-1 (stromal derived factor-1), and the VLA-4 receptor (very late antigen 4), formed through the binding of integrin β1 and α4, which interacts with vascular cell adhesion molecule 1 (VCAM-1) [9].
Tissue injury is important in the homing and regenerative effects of MSCs. This capability was demonstrated in the work of Reis et al. [10], who showed that the preventive administration of MSCs did not improve nephrotoxic acute kidney injury (AKI) and that the production of chemotactic mediators in an injured kidney is required for the homing of the cells.
The beneficial effects of exogenously administrated MSCs have been demonstrated in various models of acute and chronic kidney injury. The direct effects of MSCs at the site of injury include minimizing or preventing renal dysfunction and proteinuria in 5/6 nephrectomy and renovascular hypertension models of CKD, respectively [11, 12]. In addition, tubular dysfunction was found to be diminished, including decreased apoptotic, necrotic cell death and increased cellular proliferation, in nephrotoxic and ischemia/reperfusion models of AKI [10, 13]. MSCs have immunomodulatory properties that include the induction of decreased levels of inflammatory factors [interleukin (IL)-1, IL-6; tumor necrosis factor (TNF) alpha] and increased levels of anti-inflammatory factors (IL-10) [10, 14, 15].
MSCs can be pre-conditioned to have increased regenerative capacity. This strategy enables the cells to have improved engraftment properties and to survive in hostile microenvironments. Such pre-conditioning has been performed under various conditions. The exposure of culture-maintained bone marrow to hypoxic conditions (1% O2 for 24 h) has been found to increase hepatocyte growth factor (HGF) production, SDF-1, and CXCR4 gene expression (both encoding chemokine proteins) and to decrease pulmonary fibrosis [16] and myocardical infarction size [17].
Exogenous substances, such as antioxidants, hormones, and growth factors, have also been used to improve the engraftment and viability of MSCs at the site of tissue damage. Curcumin [18], statins [19], erythropoetin [20], growth factors, such as granulocyte-colony stimulating factor [21], and transfection with a specific microRNA [22] have also been tested to improve MSC homing and engraftment.
Research on MSC has led to the generation of three hypotheses regarding their protective/repair effect: (1) migration to the injured tissue and secretion of paracrine modulators, (2) transdifferentiation into resident cells to repopulate the tissue, and (3) fusion with resident cells. It is now evident that the mechanism of action of stem cells, at least in the kidney, is mainly paracrine. Although MSCs or other stem cells show differentiation capability, none of them differentiate into kidney epithelial cells, and thus they do not repopulate injured kidney cells through differentiation [23, 24].
Studies using several kidney injury models have demonstrated that the conditioning medium can mediate most of the effects of MSCs [10, 25, 26]. In contrast, only a few studies have shown that MSCs indeed fuse with [27] or transdifferentiate into resident cells, probably after the fusion process [28]. Thus, knowing that the main protective/regenerative effect of MSC in kidney diseases is paracrine, the next challenge is to determine which paracrine factors mediate MSC effects.
MSC paracrine factors: growth factors, cytokines, extracellular vesicles
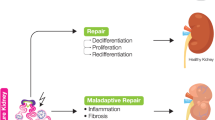
The effects of MSCs depend on the interaction of these cells with epithelial cells. This interaction may be the key factor in renal regeneration after damage (Fig. 1). The paracrine/endocrine effect of MSCs is achieved through the secretion of growth factors, cytokines, or extracellular vesicles at the site of kidney damage.
Interaction between mesenchymal stromal cells (MSCs), renal progenitor cells (RPCs), and tubular cells. One hypothesis suggests the existence of RPCs that contribute to kidney repair, and another hypothesis suggests that differentiated tubular cells participate in the repair mechanism through dedifferentiation and redifferentiation after injury. MSCs mainly contribute to the regeneration/repair process through paracrine factor secretion. VEGF Vascular endothelial growth factor, HGF hepatocyte growth factor, IGF insulin-like growth factor
The growth factors found in the stem cell secretome include HGF, insulin-like growth factor 1 (IGF-1), and vascular endothelial growth factor (VEGF) [29], all of which have angiogenic effects. VEGF production is stimulated in MSCs under hypoxic conditions, which in turn stimulates endothelial cell proliferation [30]. HGF and IGF are both produced by MSCs and show antifibrotic effects by antagonizing the transforming growth factor beta 1/SMAD pathway [31]. These growth factors are also produced by the renal cell during injury [32, 33]. Therefore, it is reasonable to assume that, under conditions of injury, the renal cells produce growth factors that participate directly in cell repair. Both the migration of MSCs to the lesion site induced by chemotactic factors and, most likely, the presence of resident stem/progenitor cells amplify this signal by stimulating the additional production of growth factors, thereby ameliorating cell repair.
Extracellular vesicles (EVs) are nano- and microparticles that are released from virtually all cells. They are classified by their size, generation mechanism, and secretion mode, including exosomes and microvesicles [34]. Their main physiological function is to mediate intercellular communication via protein, RNA, and microRNA transport to recipient cells. It has been suggested that damaged tubular cells release EVs that stimulate the homing of MSCs to the kidney. Once the MSCs arrive at the damaged organ, they release EVs to reprogram the recipient cell, allowing proliferation and repair. The main advantage of EVs is that they are not immunogenic and may mimic most of the regenerative effects of MSCs [25, 35,36,37]. MSCs can also be conditioned to produce larger amounts of EVs, which can enhance the EV effect, providing more regenerative potential.
Despite the enormous potential of EVs, their clinical use still involves certain challenges that have to be investigated, such as: What is the best EV extraction method? What is the best type of EV to use in kidney diseases, i.e., microvesicles or exosomes? What EV concentration should be used?
It is noteworthy that the secretome of stem cells has some advantages over the transplantation of the cells themselves in clinical therapeutic use. The extraction and conditioning of the secretome is easier than the administration of stem cells. Additionally, it is easier to produce the secretome on a large scale than it is to produce a population of stem cells. However, one possible hindrance to this method is that stem cells modify their secretome depending on culture conditions. In fact, to produce conditioning medium on a large scale, it is necessary to culture MSCs in a three-dimensional (3D) culture, such as in microspheres. MSCs cultured in microspheres show increased production of PGE2, a prostanoid with physiological and proinflammatory effects under cyclooxygenase activity. In MSCs, PGE2 production has immunomodulatory and anti-inflamatory effects [38].
Additionally, 3D culturing of MSCs may also modify their secretome. MSCs grown in microcarries or microspheres have been shown to produce trophic factors with antiapoptotic and anticancer proprieties, such as the anti-inflammatory molecules TSG-6 (TNF-stimulated gene 6 protein), STC-1, and CXCR4. These factors are not found in high concentrations when cells are grown in a monolayer. The anticancer molecules detected in the 3D MSC secretome are TRAIL, interleukin (IL)-24, and CD (cluster of differentiation) 82 [39, 40].
In conclusion, more studies are needed to analyze secretome modification in response to the preconditioning of MSCs and its application in treating kidney diseases.
MSC interaction with resident stem/progenitor cells: kidney progenitor stem cells
The adult mammalian kidney has a complex structure with a low regeneration rate. Nevertheless, in response to acute or moderate injury, kidney epithelial cells are replenished with new proliferative epithelial cells. One hypothesis which has been proposed to explain this phenomenon is that tubular epithelial cells interact with RPCs, but the origin of the latter is controversial.
It is proposed that there are over 24 types of mature stem cells distributed throughout vascular, interstitial, glomerular, and tubular compartments. As each type has its own markers [41], it is difficult to investigate RPC participation in organ repair. Nevertheless, some studies have been conducted, with promising results [42,43,44].
Maeshima et al. localized RPCs using a BrDU (thymidine analog) labeling strategy [42]. As kidney progenitor cells have low proliferation rates in physiological conditions, they retain the BrDu label; in contrast, the marker is diluted in other cells that proliferate more frequently [42]. Using the BrDU strategy, these authors found labeled cells scattered between tubule cells that expressed vimentin, a mesenchymal marker. However, after several cellular divisions, these cells expressed a different epithelial marker, E-cadherin, instead of vimentin, providing evidence of differentiation into epithelial cells [42].
In another study, Bussolati et al. showed that human kidney progenitor cells, extracted from the kidney cortex, express CD133 and PAX2, a kidney embryonic marker [43]. These cells were capable of expansion, limited self-renewal, and differentiation into endothelial and epithelial cells, and when administered to mice with AKI, they were able to embed in the kidney and integrate into tubules [43].
Ronconi et al. observed renal progenitor cells (RPCs) in the urinary and vascular poles of Bowman’s capsule [44]. Progenitor cells found in the urinary pole expressed CD133 and CD24 and were able to regenerate podocytes and tubular cells. However, progenitor cells localized between the vascular and urinary poles expressed CD133, CD24, and podocalyxin, a podocyte marker. These progenitor cells had the potential to regenerate only podocytes, showing that there is a hierarchy among progenitor cells. In an adriamycin model of glomerular injury, these cells were only able to regenerate podocytes, and improved glomerular injury [44].
Podocytes are cells formed during the embryonic development of the kidney that lack proliferation capacity. Wanner et al. proposed that podocytes can be formed from stem cells localized at the parietal membrane of Bowman’s capsule [45]. These authors classified RPCs positive for CD133 and CD24, also based on positivity for the CD106 marker VCAM1. CD106-negative RPCs were found around the proximal and distal tubules, and CD106-positive RPCs were localized at the urinary pole of Bowman’s capsule. Only CD133+, CD24+ and CD106- cells were characterized as progenitor cells committed to tubular regeneration [45]. These cells have been observed to improve kidney function when administered in cases of glycerol-induced AKI in mice [46].
Nevertheless, some groups state that cells with progenitor characteristics do not exist in the adult kidney and that they are restricted to embryonic development [47]. Progenitor cell selection employing membrane markers such as CD133 or CD34 may be imprecise, possibly due to progenitor cells switching markers when submitted to cell culture conditions. Some research groups have already performed progenitor cell selection and characterization of these cells from fetal human kidneys, followed by evaluation of the effect of these cells on mouse renal disease [48]. A population of RPCs isolated from human fetal kidneys (15–23 weeks) based on expression of the NCAM1 marker showed clonogenic and tubulogenic capacity [47]. The transplantation of this cell population into a chronic renal disease model, induced by 5/6 nephrectomy, promoted late improvement in the creatinine clearance, with signs of renal epithelium induction in the remaining kidney [47,48,49].
The existence of progenitor cells in the adult kidney and their participation in kidney regeneration is as yet an unresolved question. While the results of some studies suggest the existence of a fixed population of progenitor kidney cells [43], others claim that upon kidney injury and following a specific stimulus, epithelial cells proliferate in a lineage-specific manner, restricted to their segment of the nephron [50, 51]. In this microenvironment, epithelial cells acquire membrane markers and characteristics of progenitor cells, being responsible for tubule repair, even in 3D culture conditions [24, 52, 53].
Tubular epithelial cell participation in repair/regeneration
The proximal tubule has the capacity to proliferate and regenerate after an acute injury [54]. However, this regenerative capacity is sometimes incomplete and, depending on the intensity and frequency of the injury episodes, CKD may be induced. One theory supports the notion that, after an acute injury, the surviving proximal tubular cells undergo a cycle of injury, dedifferentiation, and redifferentiation, during which they express the markers of dedifferentiated cells, such as vimentin, injury markers, such as kidney injury molecule 1 (KIM-1), and also progenitor stem cell markers, such as CD24 and CD133 [55].
Differentiated tubular cells have been shown to induce tubular regeneration [50, 51, 55, 56], but in all studies reported here describing such tubular regeneration, the participation of RPCs in kidney repair could not be demonstrated.
Applying this fate-tracing approach, Kusaba et al. genetically labeled the apical membranes of differentiated tubular epithelial cells, which express SLC34a1, sodium-dependent inorganic phosphate transporter in the S1 and S2 segments of the proximal tubule after tamoxifen treatment. The mice were then subjected to ischemia/reperfusion, and their injured, labeled tubular cells were found to express CD133, CD24, vimentin and KIM-1, all potential RPC markers. This work demonstrates that differentiated proximal tubular cells contribute to tubular repair and does not support the existence of tubular progenitor stem cells [55].
In another fate-tracing protocol, potential tubular progenitor cells, named scattered tubular cells (STCs), were genetically labeled through the transcriptional activity of β-galactosidase upon doxycycline induction [56]. Doxycycline was administered to label STCs, following which ischemia and reperfusion were induced; the transcriptional activity of β-galactosidase did not increase. In the same work, tubular cells were genetically labeled with green fluorescent protein (EGFP)-tagged histone upon doxycycline induction and, after three different types of acute injury, the number of EGFP-labeled cells increased, mainly in the proximal segment of the nephron. These results show that STCs did not contribute to proximal tubule regeneration in this model. On the contrary, upon injury, proximal tubular cells adopt a phenotype similar to that of RPCs. In this study, STCs did not represent a fixed population of progenitor cells [56].
A third study showed the participation of differentiated tubular cells in kidney repair [50]. In this study, a tubular cell was genetically labeled with one of four types of color by crossing Rainbow mice with mice harboring an inducible Cre-ER fusion protein under the control of the ubiquitous actin promoter. Tamoxifen injection induced a fusion protein in 12-week-old mice offspring [50] which entered the nucleus and randomly recombined with one of the four colors in each actin-expressing cell. Although the cells were sparsely colored under low doses of tamoxifen, after 7 months lineage tracing showed regions of a single color in all kidneys, showing that each colored region was formed by the proliferation of a single cell. Similar results were observed after ischemia/reperfusion injury [50]. Tamoxifen administration before ischemia/reperfusion injury led to similar results 2 months after injury. Single-colored clones, restricted to their segment of the nephron, appeared in the damaged kidney cortex, medulla, and papilla. The clones proliferated but did not cross to other segments of the nephron. These results demonstrate that single clones contribute to tubulogenesis after damage [50].
These studies highlight the participation of the tubular cell in the repair process after injury via dedifferentiation and redifferentiation. It is therefore reasonable to suggest that several mechanisms act independently in the regeneration process, such as stromal cell paracrine effects, kidney progenitor cell regenerative effects, and tubular cell proliferation. Additionally, the kidney presents other cells, such as endothelial, vascular, and stromal cells, which may be crucial to the regenerative process [57, 58]. However, this process can be more effective and faster when multiple mechanisms are working at the same time, in a synergistic manner.
Rebuilding the entire organ for transplant
Zebrafish kidney development model may guide studies on human kidney regeneration
It seems difficult to understand the relationship between human kidney regenerative medicine and zebrafish studies. Nevertheless, tubular regeneration in response to moderate injury in fish is very similar to that seen in mammals [59]. Tubular lesion leads to the denudation of the tubule and the exposure of the basement membrane, which is replaced by cuboidal cells that maturate to restore the function of the nephron [59]. Not only is fish kidney regeneration similar to that observed in the mammalian kidney in response to injury, but also nephron hypertrophy, an event that occurs after kidney injury in the adult human kidney, is observed [60].
Zebrafish studies have many advantages, of which one of the most important is the occurrence of neo-nephrogenesis. Nephrogenesis only occurs in mammals during embryonic development, when new nephrons are formed. However, nephrogenesis, referred to as neo-nephrogenesis, does occur in adult fish in response to injury-recapitulating nephrogenesis [59]. Studies of the genes and transduction products involved in neo-nephrogenesis may contribute to the development of regenerative human therapies because several pathways are evolutionarily conserved between species, as are the genes and translation products that mediate physiological processes. Additionally, the genome sequence and protein-coding gene functions in zebrafish have already been identified, allowing researchers to identify homologous genes between zebrafish and humans [61].
Zebrafish larvae may also be employed to study not only kidney development but also to determine how some pathologies affect the nephron. Indeed, zebrafish larvae possess a structure similar to that of the mammalian nephron, i.e., a pronephros [62]. Specialized cells, such as podocytes and endothelial cells from fenestrated capillaries, are observed in zebrafish glomeruli, and polarized epithelial cells are observed in the pronephric tubules [62]. Zebrafish larvae podocytes express nephrin and podocin, proteins that interact within the slit diaphragm structure. Kidney diseases characterized by massive proteinuria involve impairment of the slit diaphragm protein expression of nephrin, as observed in diabetic nephropathy [63].
The study of tubule development in zebrafish may help to elucidate various human kidney pathologies. In zebrafish, tubule development is mediated by the transcription factor hepatocyte nuclear factor 1β (HNF-1β) [64]. The mutation of this factor leads to glomerular cyst formation. In humans, HNF-1β mutations are associated with glomerulocystic disease and maturity-onset diabetes of the young (MODY) [65]. A better understanding of HNF-1β transcription factor in zebrafish will help researchers to understand glomerulocystic disease in humans.
The zebrafish larval pronephros has been used to study nephrotoxic AKI. Exposure to gentamicin induces alterations in the pronephric kidney which are similar to those observed in the mammalian kidney: loss of the tubule brush border, tubular flattening, tubule lumen debris accumulation, and loss of cell polarity [66].
Exposure to nephrotoxins stimulates neo-nephrogenesis in zebrafish, as observed in a gentamicin nephrotoxic model where neo-nephrogenesis was initiated by a cluster of Lhx1a (Lim1 homeobox protein)-positive cells [67]. These cells express the PAX-2 gene, which is also present in mammalian kidney progenitor stem cells. It has been suggested that Lhx1a-positive cells may be the earliest marker of nephron progenitor cells in adult zebrafish [67]. Altogether, zebrafish progenitor cells are a strong candidate to elucidate the possible niches and markers of RPCs in the human kidney.
AKI in humans is characterized by kidney function loss and increases in plasma creatinine and urea levels. Kidney function is difficult to characterize in zebrafish, but clinical signs, such as low hematocrit values and hypoproteinemia can help to characterized kidney injury in fish [68].
Other advantages of zebrafish studies are the development of the zebrafish embryo outside the mother’s body, in fresh water, the transparency of the larvae, allowing morphological changes due to mutations to be more easily identified [62], the small size of adult zebrafish , enabling large numbers of animals to be kept in a small space, and the high breeding rate, yielding a high number of progeny. Fertilized eggs also develop into swimming larvae within 2.5 days.
In order to better study human genes involved in diseases, genetic approaches have already been developed using zebrafish, such as morpholino knockdown and interference RNA knockout mice [61].
Tissue bioengineering: repopulation of kidney scaffolds and development of organoids
The great advantage of tissue bioengeneering consists in the possibility of generating a complex organ ex vivo, such as a kidney, and transplanting this functional organ into a patient suffering from CKD. Many of the challenges of building an ex-vivo kidney have been solved to some extent. First, the production of decellularized kidney matrix has already been successfully accomplished through the use of detergents such as Triton-X 100 and sodium dodecyl sulfate (SDS) [69]. Decellularization with 1% SDS seems to be the most effective method, keeping the matrix closest to its original structure [69]. Acellular structures have been achieved in human, rat, and porcine kidneys [70].
The study of the decellularized matrix structure and its architecture are important for two reasons: first, determining the matrix architecture is crucial, so that 3D printers, using biomaterials such as collagen fibers, another step toward ex-vivo kidney construction, could mimic a kidney with high fidelity. Second, differences in the extracellular matrix architecture in different regions of the kidney can determine cellular morphology and differentiation [71].
Another challenge concerns scaffold reseeding. The decellularized extracelular matrix must be repopulated by cells that give rise to cells with differentiated phenotypes and specific functions. Several studies have already shown that this is possible [70, 72]. One example is the use of pluripotent embryonic stem cells (ESCs) administered through the renal artery or the ureter to reseed the renal scaffold [72].
Many challenges still must be overcome in kidney bioengeneering, but new perspectives have emerged with the production of kidney organoids. These include human pluripotent stem cells (hPSCs) that have been reprogrammed in vitro to produce kidney organoids [73]. The hPSCs were cultured in matrigel to form epiblast spheroids, recapitulating embryogenesis. The epiblast spheroids were differentiated into descendant epithelia through the inhibition of glycogen synthase kinase-3β and incubation with commercially available neuronal supplement media [73, 74]. Under culture conditions, the epiblast spheroids created tubular organoids within 10 days. The tubular organoids retained tubular cell markers such as Lotus tetragonolobus lectin (LTL), which reacts with proximal tubular structures. Megalin and cubulin were co-expressed and localized with LTL. Podocyte markers, such as Wilms tumor protein and synaptopodin, were also expressed in the organoids. CD31 and von Willebrand factor were expressed, suggesting a vascular structure and a nephron-like structure in the organoids. Additionally, under nephrotoxic exposure induced by gentamicin and cisplatin, tubular organoids upregulated KIM-1, a well-known tubular injury marker. Similar results have been obtained in other studies [75, 76].
The study by Morizane et al. [73] opens a new perspective in regenerative medicine as a PSC will be able to originate an entire functional organ. More importantly, PSCs can be created by reprogramming the somatic cells of a patient through the expression of four genes (Oct4, SOX2, c-Myc, and Nanog), to form the induced PSCs (iPSCs) [77] needed to generate kidney organoids.
More impressively, potential genetic defects in these somatic cells can be corrected using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 genome editing system technology, allowing a non-immunogenetic kidney to be built from the patient’s own cells (Fig. 2). The possibilities are endless; however, there is a long way to go before these organoids can give rise to a fully formed kidney.
Conclusion and future perspectives
Nowadays, there are several alternative methods that can be used in kidney regenerative medicine. Although these are distinct, with the advancement of science and the generation of new knowledge, they are becoming complementary to each other. Pluripotent (iPSCs, ESCs), multipotent (MSCs) and adult (kidney progenitor) stem cells and, possibly, their paracrine factors (exosomes, growth factors), in addition to the proximal tubular cell itself, actively participate in kidney regeneration processes. Chronic or more extensive injury, requiring RRT, will be probably treated with the construction of a new organ from a renal organoid or by repopulation of a scaffold using the recipient’s own cells, which would presumably avoid rejection.
In summary, we are close to the day when we will re-edit a somatic cell from a patient through gene editing technology and use it to produce an induced PSC and the patient’s own nephrons, recapitulating zebrafish neo-nephrogenesis. This cell will repopulate a kidney scaffold produced on a 3D printer or, alternatively, will develop an organoid and a functional kidney.
References
Sugai K, Fukuzawa R, Shofuda T, Fukusumi H, Kawabata S, Nishiyama Y, Higuchi Y, Kawai K, Isoda M, Kanematsu D, Hashimoto-Tamaoki T, Kohyama J, Iwanami A, Suemizu H, Ikeda E, Matsumoto M, Kanemura Y, Nakamura M, Okano H (2016) Pathological classification of human iPSC-derived neural stem/progenitor cells towards safety assessment of transplantation therapy for CNS diseases. Mol Brain 9:85
Fong C-Y, Gauthaman K, Bongso A (2010) Teratomas from pluripotent stem cells: a clinical hurdle. J Cell Biochem 111:769–781
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Ren H, Sang Y, Zhang F, Liu Z, Qi N, Chen Y (2016) Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int 2016:3516574
Squillaro T, Peluso G, Galderisi U (2016) Clinical trials with mesenchymal stem cells: an update. Cell Transplant 25:829–848
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A (2005) Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy 7:393–395
Butcher EC, Picker LJ (1996) Lymphocyte homing and homeostasis. Science 272:60–66
Segers VF, Van Riet I, Andries LJ, Lemmens K, Demolder MJ, De Becker AJ, Kockx MM, De Keulenaer GW (2006) Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am J Physiol Heart Circ Physiol 290:H1370–H1377
Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N (2012) Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One 7:e44092
Caldas HC, de Paula Couto TA, Fernandes IM, Baptista MA, Kawasaki-Oyama RS, Goloni-Bertollo EM, Braile DM, Abbud-Filho M (2015) Comparative effects of mesenchymal stem cell therapy in distinct stages of chronic renal failure. Clin Exp Nephrol 19:783–789
Oliveira-Sales EB, Maquigussa E, Semedo P, Pereira LG, Ferreira VM, Câmara NO, Bergamaschi CT, Campos RR, Boim MA (2013) Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PLoS One 8:e78464
Zhuo W, Liao L, Xu T, Wu W, Yang S, Tan J (2011) Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int 86:191–196
Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R (2009) Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 183:993–1004
Wise AF, Williams TM, Kiewiet MB, Payne NL, Siatskas C, Samuel CS, Ricardo SD (2014) Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am J Physiol Ren Physiol 306:F1222–F1235
Lan YW, Choo KB, Chen CM, Hung TH, Chen YB, Hsieh CH, Kuo HP, Chong KY (2015) Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther 6:97
Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L (2008) Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135:799–808
Liu J, Zhu P, Song P, Xiong W, Chen H, Peng W, Wang S, Li S, Fu Z, Wang Y, Wang H (2015) Pretreatment of adipose derived stem cells with Curcumin facilitates myocardial recovery via antiapoptosis and angiogenesis. Stem Cells Int 2015:638153
Li N, Yang YJ, Qian HY, Li Q, Zhang Q, Li XD, Dong QT, Xu H, Song L, Zhang H (2015) Intravenous administration of atorvastatin-pretreated mesenchymal stem cells improves cardiac performance after acute myocardial infarction: role of CXCR4. Am J Transl Res 7:1058–1070
Lu H, Wu X, Wang Z, Li L, Chen W, Yang M, Huo D, Zeng W, Zhu C (2016) Erythropoietin-activated mesenchymal stem cells promote healing ulcers by improving microenvironment. J Surg Res 205:464–473
Yu J, Liu XL, Cheng QG, Lu SS, Xu XQ, Zu QQ, Liu S (2016) G-CSF and hypoxic conditioning improve the proliferation, neural differentiation and migration of canine bone marrow mesenchymal stem cells. Exp Ther Med 12:1822–1828
Dakhlallah D, Zhang J, Yu L, Marsh CB, Angelos MG, Khan M (2015) MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J Cardiovasc Pharmacol 65:241–251
Dekel B, Shezen E, Even-Tov-Friedman S, Katchman H, Margalit R, Nagler A, Reisner Y (2006) Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells 24:1185–1193
Dziedzic K, Pleniceanu O, Dekel B (2014) Kidney stem cells in development, regeneration and cancer. Semin Cell Dev Biol 36:57–65
Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G (2012) Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7:e33115
Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK (2010) Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 38:215–224
Held PK, Al-Dhalimy M, Willenbring H, Akkari Y, Jiang S, Torimaru Y, Olson S, Fleming WH, Finegold M, Grompe M (2006) In vivo genetic selection of renal proximal tubules. Mol Ther 13:49–58
Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW (2002) Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416:542–545
Nigam eS, Lieberthal W (2000) Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Ren Physiol 279:F3–F11
Razban V, Lotfi AS, Soleimani M, Ahmadi H, Massumi M, Khajeh S, Ghaedi M, Arjmand S, Najavand S, Khoshdel A (2012) HIF-1α Overexpression induces angiogenesis in Mesenchymal stem cells. Biores Open Access 1:174–183
Xing L, Cui R, Peng L, Ma J, Chen X, Xie RJ, Li B (2014) Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Res Ther 5:101
Dai C, Liu Y (2004) Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol 15:1402–1412
Mizuno S, Matsumoto K, Kurosawa T, Mizuno-Horikawa Y, Nakamura T (2000) Reciprocal balance of hepatocyte growth factor and transforming growth factor-beta 1 in renal fibrosis in mice. Kidney Int 57:937–948
Borges FT, Reis LA, Schor N (2013) Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res 46:824–830
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G (2009) Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20:1053–1067
Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y (2014) Microvesicles derived from human Wharton’s jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther 5:40
Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H (2016) Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res 20:21
Bouffi C, Bony C, Courties G, Jorgensen C, Noël D (2010) IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One 5:e14247
Bartosh TJ, Ylöstalo JH, Bazhanov N, Kuhlman J, Prockop DJ (2013) Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1). Stem Cells 31:2443–2456
Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ (2010) Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their anti-inflammatory properties. Proc Natl Acad Sci USA 107:13724–13729
Dressler GR (2006) The cellular basis of kidney development. Annu Rev Cell Dev Biol 22:509–529
Maeshima A, Yamashita S, Nojima Y (2003) Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 14:3138–3146
Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G (2005) Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166:545–555
Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P (2009) Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20:322–332
Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB (2014) Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol 25:707–716
Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P (2012) Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30:1714–1725
Harari-Steinberg O, Metsuyanim S, Omer D, Gnatek Y, Gershon R, Pri-Chen S, Ozdemir DD, Lerenthal Y, Noiman T, Ben-Hur H, Vaknin Z, Schneider DF, Aronow BJ, Goldstein S, Hohenstein PDB (2013) Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med 5:1556–1568
Pode-Shakked N, Pleniceanu O, Gershon R, Shukrun R, Kanter I, Bucris E, Pode-Shakked B, Tam G, Tam H, Caspi R, Pri-Chen S, Vax E, Katz G, Omer D, Harari-Steinberg O, Kalisky T, Dekel B (2016) Dissecting stages of human kidney development and tumorigenesis with surface markers affords simple prospective purification of Nephron stem cells. Sci Rep 6:23562
O’Brien LL, McMahon AP (2014) Induction and patterning of the metanephric nephron. Semin Cell Dev Biol 36:31–38
Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, Lim X, Van-Amerongen R, Bowman A, Januszyk M, Pleniceanu O, Nusse R, Longaker MT, Weissman IL, Dekel B (2014) In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep 7:1270–1283
Romagnani P, Rinkevich Y, Dekel B (2015) The use of lineage tracing to study kidney injury and regeneration. Nat Rev Nephrol 11:420–431
Buzhor E, Harari-Steinberg O, Omer D, Metsuyanim S, Jacob-Hirsch J, Noiman T, Dotan Z, Goldstein RS, Dekel B (2011) Kidney spheroids recapitulate tubular organoids leading to enhanced tubulogenic potency of human kidney-derived cells. Tissue Eng A 17:2305–2319
Buzhor E, Omer D, Harari-Steinberg O, Dotan Z, Vax E, Pri-Chen S, Metsuyanim S, Pleniceanu O, Goldstein RS, Dekel B (2013) Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. Am J Pathol 183:1621–1633
Little MH (2006) Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol 17:2390–2401
Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD (2014) Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA 111:1527–1532
Berger K, Bangen JM, Hammerich L, Liedtke C, Floege J, Smeets B, Moeller MJ (2014) Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci USA 111:1533–1538
Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, Reisner Y (2006) Isolation and characterization of nontubular sca-1+lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol 17:3300–3314
Hu Y, Li M, Göthert JR, Gomez RA, Sequeira-Lopez ML (2016) Hemovascular progenitors in the kidney require sphingosine-1-phosphate receptor 1 for vascular development. J Am Soc Nephrol 27:1984–1995
Reimschuessel R, Bennett RO, May EB, Lipsky MM (1990) Development of newly formed nephrons in the goldfish kidney following hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol 18:32–38
Fine L (1986) The biology of renal hypertrophy. Kidney Int 29:619–634
Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fényes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, Stemple DL (2013) A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496:494–497
Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC (1998) Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125:4655–4667
Benigni A, Gagliardini E, Tomasoni S, Abbate M, Ruggenenti P, Kalluri R, Remuzzi G (2004) Selective impairment of gene expression and assembly of nephrin in human diabetic nephropathy. Kidney Int 65:2193–2200
Sun Z, Hopkins N (2001) Vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev 15:3217–3229
Froguel P, Velho G (1999) Molecular genetics of maturity-onset diabetes of the young. Trends Endocrinol Metab 10:142–146
Cianciolo Cosentino C, Roman BL, Drummond IA, Hukriede NA (2010) Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp 42:3–7
Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, Davidson AJ (2011) Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470:95–100
Kamei CN, Liu Y, Drummond IA (2015) Kidney regeneration in adult Zebrafish by Gentamicin induced injury. J Vis Exp 102:e51912
Nakayama KH, Batchelder CA, Lee CI, Tarantal AF (2010) Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng A 16:2207–2216
Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC (2013) Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19:646–651
O’Neill JD, Freytes DO, Anandappa AJ, Oliver JA, Vunjak-Novakovic GV (2013) The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials 34:9830–9841
Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD (2009) Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol 20:2338–2347
Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33:1193–1200
Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman T, Zhou J, Lerou PH, Bonventre JV (2015) Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6:8715
Schutgens F, Verhaar MC, Rookmaaker MB (2016) Pluripotent stem cell-derived kidney organoids: an in vivo-like in vitro technology. Eur J Pharmacol 790:12–20
Takasato M, Er PX, Chiu HS, Little MH (2016) Generation of kidney organoids from human pluripotent stem cells. Nat Protoc 11:1681–1692
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Borges, F.T., Schor, N. Regenerative medicine in kidney disease: where we stand and where to go. Pediatr Nephrol 33, 1457–1465 (2018). https://doi.org/10.1007/s00467-017-3754-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3754-9