Abstract
Over the past several decades, the epidemiology of acute kidney injury (AKI) in children has changed significantly. Pediatric patients with AKI frequently have co-morbid conditions, substantial fluid overload, and marked disease severity. At the same time, continuous renal replacement therapy (CRRT) has become the preferred modality for the management of these patients. This manuscript provides a state-of-the-art review of the technical aspects of pediatric CRRT and examines the most recent data regarding CRRT indications, timing of initiation, dosing, and outcomes in critically ill children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, continuous renal replacement therapy (CRRT) has become the preferred modality for the management of critically ill children with acute kidney injury (AKI) and fluid overload [1, 2]. This has occurred, in part, due to advances in technology allowing more accurate treatment delivery and better control of blood flow and fluid removal. The result is steadily improving reliability and ease of operation of a therapy which offers several advantages over more traditional dialysis methods when used in critically ill or intrinsically unstable children. Removal of solutes and modification of the extracellular fluid (ECF) volume occur gradually yet continuously during CRRT and, as a result, unstable patients who are often intolerant of the abrupt volume and solute concentration changes which accompany standard hemodialysis can usually be safely treated with CRRT. While peritoneal dialysis also has the capacity to provide gradual clearance and ultrafiltration, CRRT has the ability to independently adjust composition and volume of the ECF. Additionally, CRRT has the capacity, when required, to provide far more efficient clearance than standard peritoneal dialysis treatments.
In many ways, the basic principles of CRRT are similar for adults and children. However, the application of CRRT to pediatric care requires an understanding of several considerations which are unique to therapy in pediatric patients including: extracorporeal blood volume concerns and the need for circuit blood priming, adaptation of equipment and prescriptions designed for adult-size patients, and the use of CRRT to manage conditions unique to pediatric patients, such as inborn errors of metabolism.
This paper is intended to provide an overview of the technical aspects of pediatric CRRT as now practiced in our center and others, along with a review of the most current data regarding CRRT indications, timing of initiation, dosing, and outcomes in children.
Pediatric AKI/CRRT epidemiology and demographics
Since the majority of patients receiving CRRT are critically ill children with AKI, it is important to understand AKI epidemiology, which has changed over the past several decades. Single-center reports from the 1980s highlight hemolytic uremic syndrome and other primary renal diseases, sepsis, and burns as the most common causes of pediatric AKI [3, 4]. Recent reports, however, suggest that much of the AKI seen today is secondary to other primary or systemic diseases. Two large pediatric data sets report that congenital heart disease (and corrective surgery), acute tubular necrosis (ATN), sepsis, and administration of nephrotoxic medications are now the most common causes of pediatric AKI [5, 6]. Thus, many children with AKI now commonly have one or more co-morbid conditions that may affect their clinical course and outcome. It is important to note that this epidemiologic shift has occurred primarily in developed countries where the use of CRRT is more prevalent. In developing countries, AKI continues to be caused by primary renal disease such as hemolytic uremic syndrome, glomerulonephritis, and hypovolemic ATN [7, 8].
The most robust pediatric CRRT data available come from the Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) Registry [9]. The registry contains data on children from 13 US centers with ages ranging from newborn to 25 years and weights ranging from 1.3 kg to 160 kg [10]. It is clear from these data that the provision of CRRT to children requires an adaptable approach. The ppCRRT data also report that nearly half of the patients beginning CRRT were receiving diuretics and two-thirds were receiving vasopressor support. Based on this, it is reasonable to infer that children who require CRRT commonly have substantial underlying fluid overload and marked severity of disease.
Indications for use of CRRT
The indications for initiation of CRRT are, generally speaking, similar in adults and children. Primarily it is utilized in critically ill children with AKI and fluid overload, although certain unique indications exist. Of the patients in the ppCRRT Registry, 29% received CRRT to treat isolated fluid overload, 13% to treat isolated electrolyte abnormalities, and 46% to treat fluid overload and electrolyte abnormalities. An additional 3% of patients received CRRT to eliminate the need for fluid restriction in order to allow greater fluid intake usually for nutrition or blood component therapy. Thus, over 90% of the population received CRRT to treat metabolic or fluid abnormalities directly related to AKI [10]. These abnormalities can include fluid overload, hyperkalemia, symptomatic uremia (encephalopathy, bleeding, pericarditis), profound metabolic acidosis, and other electrolyte derangements. Of the remaining patients, 4% received CRRT to treat hyperammonemia associated with an inborn error of metabolism, and 2% received CRRT to treat an intoxication or medication overdose.
Mechanisms of clearance and CRRT nomenclature
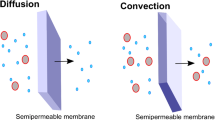
Current CRRT technology allows provision of diffusive and convective clearance, either separately or in combination. Briefly, diffusion refers to the movement of molecules down a concentration gradient across a semi-permeable membrane. Efficiency of molecular movement is inversely related to molecular size or weight and directly proportional to the magnitude of the concentration gradient. Convective clearance refers to the movement of molecules via “solvent drag”; dissolved molecules move with fluid across a semi-permeable membrane in response to a transmembrane pressure. Although diffusive and convective clearances are equally effective at small molecule removal, larger molecules theoretically move more effectively via convection.
The nomenclature of CRRT is based upon the type of vascular access and the primary method of molecular clearance. Although CRRT was initially developed based on combined arterial and venous access (i.e., CAVH, or continuous arterio-venous hemofiltration), the current technique relies upon pump-driven veno-venous access, hence the terms CVVH, CVVHD, and CVVHDF. CVVH, or continuous veno-venous hemofiltration, provides exclusively convective clearance through high ultrafiltration rates. To prevent rapid volume depletion, the majority of the ultrafiltrate is replaced with electrolyte containing fluid. In CVVHD, or continuous veno-venous hemodialysis, the majority of clearance is diffusive and occurs via countercurrent infusion of dialysate through the hemofilter. A small amount of convective clearance is provided by the net ultrafiltration used to reduce extracellular fluid volume. CVVHDF, or continuous veno-venous hemodiafiltration employs both diffusive and convective clearance. These modalities all enjoy some degree of popularity, and modality choice is usually center dependent. In an early report, the ppCRRT registry noted that 21% of patients received CVVH, 48% received CVVHD, and 30% received CVVHDF [10].
Comparisons to hemodialysis and peritoneal dialysis
As seen above, CRRT shares many principles with hemodialysis (HD) and some with peritoneal dialysis (PD). The flow rates, however, are significantly different between CRRT and hemodialysis. Frequently, CRRT utilizes slower blood and dialysate flow rates, which results in lower hourly clearance rates. CRRT compensates for this lower clearance rate by extending the clearance time. Over 24 h, CRRT can provide solute clearance comparable to that seen during a 4-h intermittent hemodialysis (IHD) session. It is quite important to highlight the different small solute clearance limitations between IHD and CRRT. Since IHD utilizes dialysate flow rates far in excess of the blood flow rate, its clearance is primarily limited by the blood flow rate. CRRT clearance, by comparison, is primarily limited by the dialysate or replacement/effluent rate. CRRT and the continuous forms of PD commonly used in the critical care setting share their continuous nature. However, CRRT technology allows for much greater daily clearance rates when compared to PD.
While a comprehensive comparison is beyond the scope of this manuscript, it may be helpful to understand the general advantages and disadvantages to each RRT therapy. The major advantages to CRRT are the ability to provide renal replacement therapy in a critically ill child while maintaining hemodynamic stability, the ability to remove a large amount of volume over an extended period of time, and the ability to nearly eliminate the need for fluid restriction, which allows provision of essential medications and blood products along with aggressive nutritional supplementation. Unfortunately, the continuous nature of CRRT which promotes hemodynamic stability can, at times, be disadvantageous since the presence of the circuit complicates the scheduling and implementation of imaging and procedures. Other disadvantages include the potential for hypothermia (addressed with a circuit heating device) and the potential to create electrolyte imbalances (addressed by addition of electrolytes to CRRT fluids and provision of high dose amino acids/protein). Although CRRT can be technically challenging in small infants and neonates, available data suggest that experienced centers can provide the therapy safely in neonates and infants <10 kg [11, 12].
Technical considerations
Although there are variations from machine to machine, many of the technical aspects of CRRT can be generalized and applied regardless of the CRRT device employed. This section will touch on those aspects while avoiding characteristics specific to individual machines.
Access
Achieving adequate vascular access, both in size and location, is essential to the delivery of CRRT. In general, larger bore catheters allow higher blood flow rates and are associated with greater CRRT circuit survival (Fig. 1a.) [13]. While longer catheters do provide greater resistance to flow, the diameter of the catheter, based on Poiseuille’s Law, has a markedly greater effect on flow than catheter length. At times, a longer catheter will allow the access to be positioned in a larger blood vessel, which results in achievement of greater blood flow rates despite the increased length; this is especially true for catheters positioned in the femoral vessels. Suggested catheter sizes based on patient weight are contained in Table 1 [14]. Although the use of two single-lumen 5-French catheters was previously proposed as a viable access solution in neonates, recently published data suggest that the use of 5-French catheters is associated with dismal circuit survival; in a study published by Hackbarth and colleagues, no circuits using two single-lumen 5-French catheters survived beyond 20 h [13]. Such poor circuit survival essentially precludes the delivery of adequate CRRT and use of such access should be discouraged [14]. Smaller bore catheters which were placed for other primary uses, such as Broviac catheters, peripherally inserted central catheters (PICC), and umbilical lines provide far too great resistance to flow and cannot be used for CRRT access. In neonates, we have had excellent technical success using 7-French catheters which are placed by the Pediatric Surgery service. Typically, they place these catheters into an internal jugular vein using a cut-down technique similar to that used for extracorporeal membrane oxygenation (ECMO) cannulation.
72-h circuit survival by catheter size (a) and insertion location (b) [13] (used with permission). a The data from the Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) demonstrates that 7- and 8-French catheters are associated with shorter continuous renal replacement therapy (CRRT) circuit survival. 5-French catheters are associated with dismal circuit survival and their use is recommended only as a last resort (data not pictured). b While catheters placed in the subclavian and femoral veins are associated with similar CRRT circuit survival, superior circuit survival is seen when catheters are placed in the internal jugular vein
Catheter location is likely to have nearly as great an effect on circuit survival as catheter size. While femoral catheters are used for CRRT nearly 3 times more frequently than internal jugular (IJ) and subclavian (SC) catheters (69 vs. 16 vs. 8%, respectively), IJ catheters lead to significantly greater circuit survival when compared to femoral or SC catheters (Fig. 1b) [13]. Many practitioners prefer femoral catheters due to ease of placement; however, bedside ultrasound devices have become more commonplace and their use greatly improves the ease and safety of IJ catheter insertions. Additionally, femoral catheters, unless they reside in the inferior vena cava are remarkably sensitive to patient movement and usually require the patient to be sedated and, at times, paralyzed for successful use. Nephrologists frequently avoid SC catheters since stenosis of the SC vein can preclude later vascular access creation for chronic HD, should the patient not regain renal function.
Filter/membrane
A number of hemofilters and membranes have been developed for use with CRRT. One of the membranes often discussed is the AN-69 membrane. This biocompatible membrane is made of polyacrylonitrile and has been frequently associated with the bradykinin release syndrome (BRS) when used in conjunction with a blood prime. Blood priming can be necessary in small infants when the extracorporeal volume of the circuit exceeds 10–15% of the patient’s estimated blood volume. The BRS occurs due to exposure of the blood to the AN-69 membrane which activates pre-kallikrein and Hageman factor leading to release of bradykinin, which is a powerful vasodilator. This reaction can lead to profound hypotension in infants 5–10 min after initiation of CRRT. Several strategies have been proposed to mitigate or prevent this syndrome [15, 16]. However, we feel that the best option for prevention of this phenomenon is avoidance of the AN-69 membrane altogether. Some practitioners advocate for the use of the AN-69 membranes specifically in patients with sepsis due to greater cytokine sieving coefficients when compared to other membranes [11, 17]. However, while studies have indicated that CRRT can remove cytokines and/or mediators of inflammation [17, 18], no studies have been able to confirm that cytokine removal, or implementation of CRRT for that matter, have the ability to improve survival in septic patients [19–21]. Our center changed from the AN-69 filter to a polyarylethersulfone (PAES) membrane over five years ago; since this change the bradykinin release phenomenon is no longer seen. However, PAES membranes are not available on all CRRT devices and they may not be available in pediatric specific sizes. Although we have used an adult sized PAES membrane successfully, even in children less than 10 kg, other polysulfone derivative membranes are available; in several studies these membranes, when compared with AN-69 membranes, have been associated with lower post-CRRT initiation bradykinin levels [15, 22].
Blood flow rates
Blood flow (Qb) is usually dependent on the access and CRRT machine. Smaller 7-Fr and 9-Fr catheters infrequently allow a Qb greater than 60–80 ml/min; older CRRT machines, such as the Gambro Prisma, have a maximal Qb of 180 ml/min. With this caveat, recommended blood flow rates of 3–10 ml/kg/min have been extrapolated from adult data and animal models [23]. Higher blood flow rates (10–12 ml/kg/min) are usually necessary in neonates and small infants for technical reasons when adult sized CRRT devices are utilized. For example, in a 4 kg neonate, a Qb of 40 ml/min may be necessary to generate access and return pressures adequate to prevent access disconnect alarms. We have tended to utilize a Qb of 30–80 ml/min in neonates and small infants, 50–100 ml/min in infants 10–20 kg, 100–150 ml/min in larger children, and 150–250 ml/min in adolescents.
Higher blood flow rates likely result in longer circuit lifespan due to reduced risk for intrafiber clotting. It is important to remember that a fundamental difference between CRRT and hemodialysis is that increasing CRRT Qb does not necessarily result in greater small solute clearance. Increasing Qb can, however, facilitate greater clearance by mitigating the reduced efficiency seen with pre-dilution mode CVVH or CVVHDF or by allowing a concomitant increase in the replacement or ultrafiltration rates. Many patients will not tolerate maximal blood flow at the initiation of CRRT and, in general, it is best to advance Qb to the targeted rate over 20–30 min.
CRRT solutions for dialysate and replacement fluid
Delivery of CRRT became more feasible and effective with the introduction of bicarbonate-based solutions. Previously, when lactate was used as the solution buffer, lactic acidosis, and the resultant cardiac dysfunction and hypotension, was common [24]. Several studies comparing lactate- and bicarbonate-based fluids clearly demonstrated the superiority of bicarbonate-based fluid, and now bicarbonate-based dialysate/replacement fluids are considered standard of care in adults and children [25, 26]. However, many fluids continue to contain a small, clinically insignificant amount of lactate to improve stability.
CRRT solutions also generally contain varying amounts of sodium, potassium, chloride, glucose, phosphate, calcium and magnesium; a myriad of electrolyte formulations are available from numerous manufacturers. Of note, calcium is always absent from solutions when phosphate is present and is usually absent when citrate anticoagulation is utilized. To avoid confusion, it has been our practice to stock a single brand in only a few formulations. In the clinical settings where a different electrolyte composition is required, the pharmacy can add potassium, phosphorous, magnesium, and even additional bicarbonate as needed. While we have had great success with this practice, it has the potential for pharmacy errors and may increase costs.
It is important to note the tendency over time for the composition of the CRRT fluids to determine the electrolyte levels of the patient. While a fluid low in potassium, phosphorous and magnesium may be appropriate at initiation of CRRT, depending on the CRRT prescription, within a surprisingly short time the patient will become frankly deficient in these electrolytes.
Hypophosphatemia, in particular, is remarkably common without solution supplementation, especially if higher clearance rates are targeted [20, 27]. Supplementation of the CRRT solutions with the necessary electrolytes creates a more physiologic fluid that will result in normalization of the electrolyte levels.
In the majority of situations, if one is utilizing CVVHDF, the replacement and dialysate fluids should have the same composition to reduce staff confusion and the risk for error. One significant exception is when albumin is added to the dialysate fluid. This technique can be used to remove protein bound medications in the setting of intoxication as well as substances such bilirubin [28].
Anticoagulation
Activation of the clotting cascade occurs in CRRT circuits due to contact of the circulating blood with artificial surfaces; this is exacerbated by lower blood flow rates, turbulent flow, small catheters, and high hematocrits. To prevent clotting and prolong CRRT circuit lifespan, anticoagulation is commonly employed, with unfractionated heparin and sodium citrate being most frequently utilized. The only large observational study in pediatrics demonstrated that heparin and citrate are equally efficacious with similar circuit lifespans, but suggested that bleeding complications were more common with heparin [29]. The majority of adult studies demonstrate prolonged circuit life and reduced bleeding with the use of citrate anticoagulation [30, 31]. However, since controlled studies in children are lacking, centers tend to adopt one method or the other based on local experience and practice; amongst practitioners from 13 US centers, citrate was used 56% and heparin 37% of the time [10]. An additional 7% of patients received no anticoagulation, relying on periodic saline flushes of the circuit. While a no-anticoagulation approach might be considered in patients with evidence of existing coagulopathy due to disseminated intravascular coagulation or hepatic failure, we discourage such practice. Many of these patients receive periodic fresh frozen plasma and platelet infusions which, without anticoagulation, commonly lead to clotting. Moreover, patients with hepatic failure may have a paradoxical hypercoagulable state. Thus, while either citrate or heparin can provide adequate CRRT anticoagulation, it is clear that avoidance of anticoagulation is associated with markedly inferior circuit life span and a reduced ability to deliver CRRT [29].
Heparin has been the mainstay of HD anticoagulation for decades. Thus, many pediatric CRRT programs began with and continue to rely on heparin to maintain circuit patency. Heparin is infused in the CRRT circuit pre-filter and titrated to achieve a targeted post-filter partial thromboplastin time (PTT) 1.5 to two times normal, or an activated clotting time (ACT) between 180 and 220 s. An extensive review of heparin protocols is beyond the scope of this manuscript, however, a commonly employed initial regimen begins with an initial heparin bolus of 20–30 units/kg, followed by a continuous infusion of 5–20 units/kg-h.
Sodium citrate anticoagulation during CRRT was first proposed by Mehta and Ahmad in 1990; its ease of administration and reduced side effects profile, when compared with heparin, has led to widespread acceptance [32, 33]. By infusing citrate into the arterial limb of the CRRT tubing as it leaves the catheter, calcium ions are bound to the citrate, creating regional hypocalcemia within the circuit tubing; this greatly inhibits coagulation within the circuit, since normal coagulation is calcium-dependent. Systemic hypocalcemia is prevented by infusing calcium chloride or calcium gluconate back into the patient at a central site away from the CRRT circuit. Thus, citrate anticoagulation achieves truly regional anticoagulation by affecting only the circuit, thereby eliminating the increased risk of bleeding seen with heparin. One example of a successful protocol was described by Bunchman and colleagues in 2002 [34]. Anticoagulant Citrate Dextrose Solution (ACD-A; Caridian BCT, Inc., Lakewood CO, USA) is initially infused at a rate equal in ml/h to 1.5–2 times the blood flow rate in ml/min. The rate is then titrated to target a circuit ionized calcium concentration of 0.25–0.4 mmols/l. A 0.8% calcium chloride in normal saline solution is concomitantly infused at an initial rate of 40–50% of the citrate infusion rate. The calcium infusion is titrated to maintain the desired systemic ionized calcium concentration, which is usually 1.1–1.3 mmols/l (often a bit higher in cardiac patients). Separate sliding scales are used to adjust citrate infusion rates according to the periodically measured circuit ionized calcium level and CaCl2 infusion rates according to systemic ionized calcium levels.
While regional anticoagulation by citrate leads to reduced bleeding complications compared to heparinization, practitioners need to be aware of the potential adverse effects associated with citrate anticoagulation. Citrate is metabolized primarily by the liver and skeletal muscle to bicarbonate in a ∼3:1 manner, thus, patients receiving citrate anticoagulation are prone to develop metabolic alkalosis. Fortunately, citrate is readily cleared by both diffusion and convection, and metabolic alkalosis can be forestalled by increasing the clearance rates [35]. Reducing the citrate infusion rate can also be effective. Citrate overload occurs when citrate clearance falls behind citrate delivery and may be diagnosed by monitoring the ratio of the total calcium to the ionized calcium levels [36]. During citrate overload, total calcium levels rise, and the ratio of total calcium to systemic ionized calcium levels rises precipitously. As citrate accumulation progresses, it becomes more difficult to maintain the declining systemic ionized calcium levels within normal ranges. Since citrate is largely cleared metabolically by the liver, patients with diminished liver function are at increased risk for citrate overload. While usually associated with a metabolic alkalosis, citrate overload has also been associated with metabolic acidosis [37]. Patients with severe hepatic dysfunction can be managed using citrate anticoagulation, but treatment often requires reducing the citrate infusion rate. We usually recommend an initial citrate infusion rate towards the lower end of the dosing range in patients with hepatic insufficiency who are at increased risk for citrate toxicity. Lower initial citrate infusion rates have also been recommended in neonates and small infants.
Dosing of CRRT
One of the greatest controversies in the CRRT literature over the past decade has been the concept of high volume therapy and the determination of the optimal CRRT dose. One of the earliest prospective, randomized trials of CRRT dosing in critically ill adults was published by Ronco and colleagues in 2000. This study demonstrated that a CRRT prescription which delivered 35 ml/kg/h of ultrafiltration led to improved survival in critically ill adults with AKI when compared with a prescription providing 20 ml/kg/h [38]. Since then, a few additional studies have examined the relationship between a higher prescribed/delivered dose of CRRT and outcomes, with fairly mixed results.
However, two large, multicenter, collaborative efforts which were recently published seem to have laid the question to rest for many practitioners. The VA/NIH Acute Renal Failure Network study clearly demonstrated that amongst 1,124 critically ill adults with AKI, there was no difference in 60-day survival rates between the more intensive CRRT group (35.8 ± 6.4 ml/kg-h) and the less intensive CRRT therapy (22.0 ± 6.1 ml/kg-h) [21]. Additionally, there was no difference in the number of patients still requiring RRT at 60 days. Not only was this study larger than all previous studies, but it was marked by an impressive ability to deliver at least the prescribed clearance targets. It is important to note, however, that patients were allowed to switch from CRRT to intermittent hemodialysis, depending on clinical hemodynamic stability. While this is applicable to current practice patterns, it makes it challenging to parse out the exclusive CRRT data. In contrast, the RENAL study included only patients who were receiving CVVHDF [20]. Based on the data from 1,508 adult patients, there was no survival difference at 90 days (Fig. 2) between the high-intensity group (33.4 ± 12.8 ml/kg/h) and the low-intensity group (22 ± 17.8 ml/kg/h). Additionally, there was no difference in the percentage of patients requiring RRT at 60 days. The consensus from these studies is that there is no benefit to delivering greater than 20–25 ml/kg/h of clearance, and it seems unlikely that a beneficial outcome will be achieved by attempting to push clearances ever higher.
Kaplan–Meier estimates of the probability of death [20] (used with permission). The RENAL Replacement Therapy Study demonstrated equivalent survival in patients receiving higher-intensity (33.4 ± 12.8 ml/kg/h) and lower-intensity (22 ± 17.8 ml/kg/h) continuous renal replacement therapy (CRRT)
One must understand, however, potential dose-independent factors that can lead to inadequate clearance. Each of the aforementioned studies was able to achieve a delivered dose that was 85–100% of the prescribed dose. In clinical practice, however, the delivered dose may be substantially lower than the prescribed dose. Circuit malfunction or membrane fouling, for example, can greatly reduce daily effluent rates; this is especially true if restarting the CRRT circuit is delayed or difficult. Additionally, many centers use pre-dilution CVVH or CVVHDF to reduce the risk of intra-filter hemoconcentration and mitigate circuit clotting risk. While this practice can minimize the risk of filter loss due to clotting, the pre-dilution technique can reduce overall clearance by 15–35%, depending on the dose [39, 40]. Finally, a study by Claure-Del-Granado and colleagues has examined the relationship between prescribed effluent dose and true small molecule clearance. This study suggested that using prescribed effluent volume as a proxy for clearance can overestimate true clearance by nearly 25% [41]; other studies have suggested that critically ill patients receive 30% less than their prescribed hemodialysis dose [39]. Thus, to be able to achieve an actual clearance of 20–25 ml/kg/h in practice, one may need to actually prescribe a higher dose.
If one chooses to run higher clearance rates, it is extremely important that the ramifications of this decision are understood. Higher-intensity CRRT has been associated both with the development of hypophosphatemia and with excessive amino acid losses [20, 39, 42]. Although these adverse events can be offset by adding phosphate to dialysate/replacement fluids and by increasing daily protein or amino acid intake to 2.5–3 g/kg/day, one must be aware of them. Finally, CRRT dose has a significant impact on drug dosing, and higher CRRT doses may be associated with supernormal drug clearance rates; this is especially problematic if the medication dose cannot be followed by blood level or titrated based on response. Critically ill children often receive multiple antimicrobial agents and may require vasoactive drips. For these medications, the practitioner must be aware that higher doses of CRRT will result in a reduced effective dose of most of these agents.
Inborn errors of metabolism
Dosing of CRRT in the setting of neonatal hyperammonemia and suspected inborn errors of metabolism warrants a brief discussion. These patients with acutely elevated ammonia levels (>300–400 μmol/l) require both administration of ammonia scavengers and early initiation of renal replacement therapy to lower ammonia levels; ideally, renal replacement therapy should begin as soon as the diagnosis is suspected. Patients with suspected inborn errors of metabolism should receive care at centers with the capacity to deliver either hemodialysis or CRRT; ammonia clearance with peritoneal dialysis is inadequate, and this therapy should only be used as a last resort if no other RRT modality is available. While intermittent hemodialysis has often been considered the most appropriate therapy due to its ability to achieve high diffusive clearance rates of ammonia, many centers have transitioned to CRRT as the mainstay treatment for inborn errors of metabolism associated with hyperammonemia. CRRT can be provided with regional anticoagulation (if citrate is used), can be delivered without the need for specialized nursing staff, and avoids the ammonia rebound seen with intermittent hemodialysis. If CRRT is used in this situation, it is imperative to deliver higher clearance rates, on the order of 8,000 ml/h/1.73 m2 [11]. Although we have tended to use CVVHD in these babies, CVVH, or CVVHDF are likely to be equally efficacious [11]. As previously mentioned, when higher clearance rates are prescribed, it is important to add electrolytes (phosphate, magnesium, and potassium) to the dialysate/replacement fluids to prevent their depletion.
CRRT outcomes
Patient outcomes following initiation of CRRT are largely dependent on the underlying disease state and co-morbid conditions, the indication for initiation, and a range of clinical criteria. Large observational studies in adult patients have suggested that mortality in adults with AKI severe enough to require renal replacement therapy is 50–80% [20, 21, 43]. In adults receiving CRRT, factors that have been associated with greater mortality risk are vasopressor support, mechanical ventilation, sepsis, severity of illness, failure of organs in addition to the kidney (heart, liver, GI, brain, lungs), and greater positive fluid balance [43–45].
A similar pattern emerges in children requiring CRRT (Table 2). Amongst patients in the ppCRRT registry, overall mortality was 42%; higher mortality rates were seen in patients with liver failure or liver transplant, pulmonary disease or lung transplant, and stem cell transplant (69, 55, and 55%, respectively) [11, 46]. Although a younger age was also associated with greater mortality, we believe this reflects the greater mortality seen in critically ill infants with AKI rather than a finding specific to CRRT. In fact, the ppCRRT data suggest that CRRT can effectively be delivered to children with weights less than 10 kg [11, 12].
Hayes et al, found similar mortality rates in their retrospective review of 76 pediatric CRRT patients; overall mortality was 44.7% and greater mortality was seen in patients with sepsis, multiple organ dysfunction syndrome (MODS), and greater fluid overload [47]. The ppCRRT registry data confirms increased mortality risk in patients with MODS (OR 4.7; 95th CI 2.0–10.7) and oncologic illness (OR 3.2; 95th CI 1.7–6.1) [46]. A large single-center report demonstrated that amongst children requiring CRRT, non-survivors tended to have higher Pediatric Risk of Mortality (PRISM) scores, lower blood pressures at presentation, and require greater pressor support [48]. Thus, the available data suggest that children with a greater burden of illness, more global organ involvement, and less hemodynamic stability are likely to have worse outcomes.
The association between severity of fluid overload at CRRT initiation and mortality deserves further mention. The most recent retrospective ppCRRT analysis demonstrated that greater fluid overload at CRRT initiation was independently associated with greater mortality, even after controlling for severity of illness (Fig. 3). The adjusted mortality OR for fluid overload was 1.03, suggesting a 3% increase in mortality risk with each 1% increase in fluid overload; patients with >20% fluid overload at CRRT initiation were 8.5 times more likely to die than those with <20% fluid overload [46]. In a large adult study, greater fluid overload was independently associated with greater mortality in patients with AKI who required renal replacement therapy. Furthermore, outcomes were superior when renal replacement therapy was initiated earlier, rather than later in the ICU course [44]. Thus, the available data, although primarily observational, suggest that outcomes are likely to be superior if CRRT is initiated earlier in the clinical course, rather than later, and at a lesser, rather than greater, degree of fluid overload.
Relationship between fluid overload and mortality in critically ill children receiving continuous renal replacement therapy (CRRT) [46] (used with permission). Data from the Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) demonstrate that children with greater fluid overload at CRRT initiation experienced greater mortality
ECMO outcomes
Combined therapy with ECMO and CRRT deserves special attention. Many patients who require ECMO develop AKI and fluid overload. CRRT can effectively and safely be used to treat uremia and fluid overload which is refractory to other interventions. We have experienced excellent technical success delivering the therapy directly into the ECMO circuit. The return line can be positioned downstream from the access line and both access points can be placed pre-bladder, pump, and oxygenator, which minimizes the risk of air embolism. Additionally, the heparin-based ECMO anticoagulation is more than adequate anticoagulation for the CRRT circuit and no additional anticoagulation, citrate or heparin, is required. AKI is clearly associated with mortality in patients receiving ECMO; those with AKI severe enough to require CRRT have survival rates of approximately 45% [49, 50]. However, survivors of combined ECMO/CRRT therapy have exceptional renal recovery outcomes. In two separate studies, 93 and 97% of ECMO/CRRT therapy patients recovered full renal function [49, 50]. In one of these studies, only 2/68 patients did not recover renal function; both of these patients had primary renal vasculitis [50].
Conclusions
In summary, CRRT has become the preferred modality to treat AKI and fluid overload in critically ill children. CRRT shares principles with both HD and PD, however, there are significant advantages and disadvantages to each method of renal replacement therapy which need to be clearly understood in order to best deliver care. The data available suggest that with adequate experience, centers can deliver CRRT to children across the entire spectrum of age and size both safely and effectively.
References
Belsha CW, Kohaut EC, Warady BA (1995) Dialytic management of childhood acute renal failure: a survey of North American pediatric nephrologists. Pediatr Nephrol 9:361–363
Warady BA, Bunchman T (2000) Dialysis therapy for children with acute renal failure: survey results. Pediatr Nephrol 15:11–13
Lattouf OM, Ricketts RR (1986) Peritoneal dialysis in infants and children. Am Surg 52:66–69
Williams D, Sreedhar S, Mickell J, Chan JCM (2002) Acute kidney failure: a pediatric experience over 20 years. Arch Pediatr Adolesc Med 156:893–900
Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD (2001) Pediatric acute renal failure: outcome by modality and disease. Pediatr Nephrol 16:1067–1071
Hui Stickle S, Brewer E, Goldstein S (2005) Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 45:96–101
Vachvanichsanong P, Dissaneewate P, Lim A, McNeil E (2006) Childhood Acute Renal Failure: 22-Year Experience in a University Hospital in Southern Thailand. Pediatrics 118:e786–e791
Van Biljon G (2008) Causes, Prognostic Factors and Treatment Results of Acute Renal Failure in Children Treated in a Tertiary Hospital in South Africa. J Trop Pediatr 54:233–237
Goldstein SL, Somers MJG, Brophy PD, Bunchman TE, Baum M, Blowey D, Mahan JD, Flores FX, Fortenberry JD, Chua A, Alexander SR, Hackbarth R, Symons JM (2004) The Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) Registry: design, development and data assessed. Int J Artif Organs 27:9–14
Symons JM, Chua AN, Somers MJG, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL (2007) Demographic Characteristics of Pediatric Continuous Renal Replacement Therapy: A Report of the Prospective Pediatric Continuous Renal Replacement Therapy Registry. Clin J Am Soc Nephrol 2:732–738
Goldstein S (2011) Continuous renal replacement therapy: mechanism of clearance, fluid removal, indications and outcomes. Curr Opin Pediatr 23:181–185
Symons J, Brophy P, Gregory M, McAfee N, Somers MJG, Bunchman T, Goldstein S (2003) Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis 41:984–989
Hackbarth R, Bunchman TE, Chua AN, Somers MJ, Baum M, Symons JM, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Alexander SR, Mahan JD, McBryde KD, Benfield MR, Goldstein SL (2007) The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs 30:1116–1121
Goldstein S (2011) Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial 24:187–191
Hackbarth R, Eding D, Gianoli Smith C, Koch A, Sanfilippo D, Bunchman T (2005) Zero balance ultrafiltration (Z-BUF) in blood-primed CRRT circuits achieves electrolyte and acid-base homeostasis prior to patient connection. Pediatr Nephrol 20:1328–1333
Pasko D, Mottes T, Mueller B (2003) Pre dialysis of blood prime in continuous hemodialysis normalizes pH and electrolytes. Pediatr Nephrol 18:1177–1183
Peng Y, Yuan Z, Li H (2005) Removal of inflammatory cytokines and endotoxin by veno-venous continuous renal replacement therapy for burned patients with sepsis. Burns 31:623–628
Bellomo R, Tipping P, Boyce N (1993) Continuous veno-venous hemofiltration with dialysis removes cytokines from the circulation of septic patients. Crit Care Med 21:522–526
Zhongheng Z, Xiao X, Hongyang Z (2010) Intensive- vs less-intensive-dose continuous renal replacement therapy for the intensive care unit-related acute kidney injury: a meta-analysis and systematic review. J Crit Care 25:595–600
Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638
Palevsky P, Zhang J, O'Connor T, Chertow G, Crowley S, Choudhury D, Finkel K, Kellum J, Paganini E, Schein RMH, Smith M, Swanson K, Thompson BT, Vijayan A, Watnick S, Star R, Peduzzi P (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20
Stoves J, Goode NP, Visvanathan R, Jones CH, Shires M, Will EJ, Davison AM (2001) The bradykinin response and early hypotension at the introduction of continuous renal replacement therapy in the intensive care unit. Artif Organs 25:1009–1013
Werner HA, Herbertson MJ, Seear MD (1994) Functional characteristics of pediatric veno-venous hemofiltration. Crit Care Med 22:320–325
Davenport A, Will EJ, Davison AM (1991) Hyperlactataemia and metabolic acidosis during haemofiltration using lactate-buffered fluids. Nephron 59:461–465
Zimmerman D, Cotman P, Ting R, Karanicolas S, Tobe SW (1999) Continuous veno-venous haemodialysis with a novel bicarbonate dialysis solution: prospective cross-over comparison with a lactate buffered solution. Nephrol Dial Transplant 14:2387–2391
Barenbrock M, Hausberg M, Matzkies F, de la Motte S, Schaefer RM (2000) Effects of bicarbonate- and lactate-buffered replacement fluids on cardiovascular outcome in CVVH patients. Kidney Int 58:1751–1757
Santiago M, López-Herce J, Urbano J, Bellón J, del Castillo J, Carrillo A (2009) Hypophosphatemia and phosphate supplementation during continuous renal replacement therapy in children. Kidney Int 75:312–316
Ringe H, Varnholt V, Zimmering M, Luck W, Gratopp A, Knig K, Reich S, Sauer I, Gaedicke G, Querfeld U (2011) Continuous veno-venous single-pass albumin hemodiafiltration in children with acute liver failure. Pediatr Crit Care Med 12:257–264
Brophy P, Somers MJG, Baum M, Symons J, McAfee N, Fortenberry J, Rogers K, Barnett J, Blowey D, Baker C, Bunchman T, Goldstein S (2005) Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant 20:1416–1421
Kutsogiannis D, Gibney RTN, Stollery D, Gao J (2005) Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int 67:2361–2367
Hetzel G, Schmitz M, Wissing H, Ries W, Schott G, Heering P, Isgro F, Kribben A, Himmele R, Grabensee B, Rump L (2011) Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: a prospective randomized multicentre trial. Nephrol Dial Transplant 26:232–239
Mehta RL, McDonald BR, Aguilar MM, Ward DM (1990) Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int 38:976–981
Ahmad S, Yeo KT, Jensen WM, Landicho D, Gregory B, Moritz JL, Kenny M (1990) Citrate anticoagulation during in vivo simulation of slow hemofiltration. Blood Purif 8:177–182
Bunchman T, Maxvold N, Barnett J, Hutchings A, Benfield M (2002) Pediatric hemofiltration: Normocarb dialysate solution with citrate anticoagulation. Pediatr Nephrol 17:150–154
Chadha V, Garg U, Warady B, Alon U (2002) Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr Nephrol 17:819–824
Meier Kriesche HU, Gitomer J, Finkel K, DuBose T (2001) Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit Care Med 29:748–752
Cerd J, Tolwani A, Warnock D (2011) Critical care nephrology: management of acid-base disorders with CRRT. Kidney Int. doi:10.1038/ki.2011.243
Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G (2000) Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30
Schiffl H (2010) The dark side of high-intensity renal replacement therapy of acute kidney injury in critically ill patients. Int Urol Nephrol 42:435–440
Parakininkas D, Greenbaum L (2004) Comparison of solute clearance in three modes of continuous renal replacement therapy. Pediatr Crit Care Med 5:269–274
Granado R, Macedo E, Chertow G, Soroko S, Himmelfarb J, Ikizler TA, Paganini E, Mehta R (2011) Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol 6:467–475
Zappitelli M, Goldstein S, Symons J, Somers MJG, Baum M, Brophy P, Blowey D, Fortenberry J, Chua A, Flores F, Benfield M, Alexander S, Askenazi D, Hackbarth R, Bunchman T (2008) Protein and calorie prescription for children and young adults receiving continuous renal replacement therapy: a report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Crit Care Med 36:3239–3245
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning ft, Investigators ESTftK (2005) Acute Renal Failure in Critically Ill Patients. JAMA 294:813-818
Payen D, de-Pont A, Sakr Y, Spies C, Reinhart K, Vincent J (2008) A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 12(3):R74
Ostermann M, Chang R (2009) Correlation between parameters at initiation of renal replacement therapy and outcome in patients with acute kidney injury. Crit Care 13(6):R175
Sutherland S, Zappitelli M, Alexander S, Chua A, Brophy P, Bunchman T, Hackbarth R, Somers MJG, Baum M, Symons J, Flores F, Benfield M, Askenazi D, Chand D, Fortenberry J, Mahan J, McBryde K, Blowey D, Goldstein S (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325
Hayes L, Oster R, Tofil N, Tolwani A (2009) Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 24:394–400
Fernández C, López-Herce J, Flores J, Galaviz D, Rupérez M, Brandstrup K, Bustinza A (2005) Prognosis in critically ill children requiring continuous renal replacement therapy. Pediatr Nephrol 20:1473–1477
Meyer R, Brophy P, Bunchman T, Annich G, Maxvold N, Mottes T, Custer J (2001) Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med 2:238–242
Paden M, Warshaw B, Heard M, Fortenberry J (2011) Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med 12:153–158
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sutherland, S.M., Alexander, S.R. Continuous renal replacement therapy in children. Pediatr Nephrol 27, 2007–2016 (2012). https://doi.org/10.1007/s00467-011-2080-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-2080-x