Abstract
To document trends and covariates of creatinemia (Scr) in extremely low birth weight (ELBW, < 1,000 g) neonates, maternal characteristics [betamethasone, premature preterm rupture of membranes (PPROM), pre-eclampsia, maternal Scr], characteristics at delivery [gestational age (GA), birth weight (BW), small for GA (SGA), Apgar, intubation] and during neonatal stay [ventilation, oxygen, parenteral nutrition, ibuprofen, steroids, intraventricular hemorrhage, retinopathy of prematurity (ROP), phototherapy] were linked with Scr observations. Data were reported by median and range or incidence. Characteristics in ELBW neonates with raised peak Scr (>P75) were compared to controls (<P75). In 151 ELBW neonates, an initial increase in Scr was observed, resulting in a peak Scr on day 3 or 4 of 99.9 (46.8–221.8) μmol/l with subsequent decrease. In cases (n = 37) with a peak Scr >P75 (112.3 μmol/l), Scr remained elevated until day 28. Mothers of cases received less betamethasone, neonates had a lower GA, lower BW, lower Apgar, and needed more often intubation. Postnatal ventilation, oxygen, parenteral nutrition, ibuprofen, steroids, ROP, and intraventricular hemorrhage were different. GA and ventilation or Apgar were independent factors for raised peak Scr. ELBW neonates display trends similar to heavier neonates, but peak Scr is higher, and the subsequent decrease slower. Raised creatinemia in ELBW neonates reflects immaturity (GA) and morbidity (ventilation, Apgar).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The recognition of acute renal failure (ARF) in preterm neonates is of relevance to adapt medical treatment during neonatal stay [1–5]. In addition, there are reports on the association of renal dysfunction in later life after extreme preterm birth, suggesting that renal impairment in early life may hereby serve as an indicator for focused renal follow-up [6–10]. The incidence of ARF in neonates, however, is extremely variable due to the absence of a robust definition of renal impairment in neonates and due to the use of cut-off values for serum creatinine (Scr) despite maturational differences within the preterm age [1, 2]. Cataldi et al. used such cut-off values and defined ARF as any value of Scr ≥ 1.3 mg/dl (114.9 μmol/l) or ≥ 1.0 mg/dl (88.4 μmol/l) in patients of < 33 weeks or ≥ 33 weeks gestational age (GA), respectively, to assess risk factors for ARF in a case-control approach [1]. These cut-off values resulted in a very high incidence (79%) in ‘biochemical’ ARF in very low birth weight (VLBW, i.e., <1,500 g) infants. Cuzzolin et al. used the same threshold values to document risk factors for ARF and identified maternal non-steroidal anti-inflammatory drugs (NSAIDs), intubation at birth, low Apgar score, respiratory distress syndrome, and neonatal ibuprofen treatment as independent risk factors for ARF in a cohort of neonates with a gestational age between 22 and 36 weeks [2].
Using paired urine collections and Scr observations in very preterm infants (27–31 weeks GA), Vieux et al. recently published normal values and the range of creatinine clearance in the first month of postnatal life. Creatinine clearance related to GA and postnatal age (PNA), resulting in a fivefold increase (7.9 to 37.9 ml/min.1.73 m3) in the first 28 days of life [11]. Although such an approach takes maturational dynamics more into account, it is more laborious because paired urine collection and Scr are needed to estimates GFR [11, 12]. In addition, Scr at birth still reflects maternal creatinine clearance, while in early neonatal life, Scr does not yet fully reflect neonatal GFR because of passive tubular back leak and maturational increase in tubular secretion [12].
We therefore aimed to describe postnatal Scr trends in a cohort of extreme low birth weight (ELBW, i.e., <1,000 g) infants. We subsequently introduced a more dynamic definition to document covariates of raised creatinemia within the ELBW population by comparing clinical characteristics in ELBW neonates with a peak Scr above the 75th percentile to ELBW neonates with a peak Scr below the 75th percentile. Using a similar approach, clinical characteristics in ELBW neonates with a peak Scr above the 90th percentile were compared with ELBW neonates below the 90th percentile.
Methods
Study population and data collection
All ELBW (i.e., birth weight < 1,000 g) newborns admitted to the neonatal intensive care unit (NICU) of the University Hospitals Leuven during a 5-year period (2000–2005) at birth were included except for neonates with congenital renal anomalies and/or early neonatal death (i.e., ≤ day 7). Data were retrospectively extracted from maternal and neonatal chart review.
Maternal chart review was searched for premature preterm rupture of membranes (PPROM), the use of tocolytics, the presence of pre-eclampsia, chorioamnionitis and administration (treatment initiated, i.e., first dose administered to the mother) of prenatal betamethasone. Maternal Scr was recorded if available in a time window of 24 h before or following delivery. Neonatal chart review was searched for characteristics at birth [BW, GA, Apgar score, the need for endotracheal intubation, gender and being small for gestational age (SGA)] and for characteristics of morbidity during subsequent neonatal stay [duration of ventilation (days), additional oxygen need (days), postnatal steroid or ibuprofen administration (yes/no), duration until full enteral feeding (days), retinopathy of prematurity (ROP ≥ grade 3), intraventricular hemorrhage (IVH, any), and phototherapy (yes/no)].
Ibuprofen was administered when a patent ductus arteriosus was clinically suspected and confirmed by cardiac ultrasound and therefore reflects an ‘early symptomatic approach’. All ELBW infants received methylxanthines (caffeine) during their neonatal stay. To qualify as growth restricted (SGA), the birth weight was compared to the birth weight of a Flemish cohort of preterm neonates as published by the Study Center of Perinatal Epidemiology to document neonates being SGA, i.e., below the 10th percentile similar to that reported earlier [13]. Based on routine procedures in our unit, phototherapy in ELBW neonates is initiated when unconjugated bilirubinemia is above 85.5 μmol/l (equal to 5 mg/dl), much lower compared to the threshold applied in term sick neonates (256.5 μmol/l, equal to 15 mg/dl).
Serum creatinine values were extracted from the available charts daily for the first 9 days of postnatal age (day 1–9, peak Scr) and further on week 2 (day 12–16), week 3 (day 18–24), week 4 (day 25–31), and week 6 (day 39–45) of postnatal age. All Scr measurements were based on the Jaffe reaction method.
Statistical analysis
Population characteristics and Scr values were described by median and range. Postnatal differences and trends in Scr for consecutive time points were evaluated based on interday paired analysis (Wilcoxon). The impact of maternal Scr on neonatal Scr was assessed using correlation (rank) and Bland–Altman approach to look for systematic or consistent bias between maternal and neonatal observations. Univariate analysis (Mann–Whitney U test, Chi-square test) was applied to search for covariates of peak Scr. Significant covariates of this univariate analysis were subsequently entered in a forward stepwise multiple regression to described the independent covariates of peak Scr.
Finally, based on a dichotomous approach of the peak Scr (either >75th percentile or <75th percentile), a logistic regression model was used to document odds ratios for ELBW patients with raised creatinemia (i.e., peak Scr > 75th percentile). A similar exercise was performed using a dichotomous approach with the peak Scr either > or < the 90th percentile. Statistical analysis was performed using Medcalc® program (Mariakerke, Belgium). A p value <0.05 was considered significant.
Results
Postnatal trends in creatinemia and link with maternal creatinemia
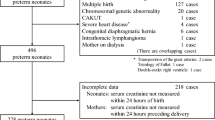
The clinical characteristics of 151 ELBW neonates and 1,883 Scr observations were available for this analysis. The clinical characteristics of ELBW neonates are provided in Table 1. The number of Scr observations and the median inter quartile (P25, P75), P90 and range of Scr for the consecutive time points are provided in Table 2. There was a significant progressive increase in median Scr for the first three consecutive days after birth (66.3, 88.4, and 96.4 μmol/l, all at least p < 0.05), without further significant difference between day 3 and 4 (96.4 vs. 95.4 μmol/l). Consequently, the median peak Scr of 99.9 (range 46.8–221.8) μmol/l for individual patients was observed on day 3 or day 4, the 75th percentile of peak Scr values was 112.3 μmol/l, the 90th percentile 128.2 μmol/l. Postnatal trends in median Scr values in ELBW (< 1,000 g) from the peak values onwards were plotted to compare with median Scr values in neonates with birth weights of 1,000–1,500 g, 1,500–2,000 g, or 2,000–2,500 g, as published earlier by Guignard et al. (Fig. 1) [4].
Median creatinemia levels in low birth weight neonates from peak creatinemia until the fourth week of postnatal life are provided for different weight categories. The observations in the cohorts of 1,000–1,500 g, 1,501–2,000 g, 2,001–2,500 g, and term neonates were extracted from the paper of Guignard et al [4] (grey discontinuous line), the observations in ELBW neonates (i.e., <1,000 g) (black continuous line) were added (X-axis = postnatal age, weeks ; Y-axis = serum creatinine, μmol/l)
Observations on maternal Scr were available in 80 mother-neonate pairs. When compared to the Scr of their neonates, a significant correlation between maternal and neonatal Scr was documented on day 1 up to day 3 (r = 0.72, 0.37, and 0.21, respectively, all at least p < 0.05). However, when using a Bland–Altman approach, the fit was only perfect at day 1 (mean diff 1.6 μmol/l, Fig. 2a), with a subsequent weakening on day 2 (mean diff 20.3 μmol/l) and 3 (mean diff 24.4 μmol/l, Fig. 2b) due to an increase in neonatal Scr compared to the maternal Scr values.
Differences between maternal and neonatal serum creatinemia on day 1 (a) and day 3 (b) of postnatal life assessed by Bland–Altman approach to illustrate the progressive increasing differences between maternal and neonatal creatinemia in the first days of postnatal life. A significant correlation between maternal and neonatal Scr was documented up to day 3 (r = 0.72, 0.37, and 0.21, respectively, all at least p < 0.05)
Covariates of Scr in ELBW neonates
Raised creatinemia, defined in the Methods section as a peak Scr above the 75th percentile (112.3 μmol/l), was documented in 37 ELBW neonates. Compared to the controls (n = 114, peak Scr < 75th percentile), Scr between neonates with a peak Scr > P75 and controls were not yet different on day 1 (p = 0.24), but remained significantly higher from day 2 until day 9 (p < 0.0001), on day 14 (p < 0.0001), 21 (p < 0.0001), and 28 (p < 0.001), but no longer on day 42 (Fig. 3). The differences in clinical characteristics [maternal, at birth and in neonatal life] between ELBW neonates with raised creatinemia (peak Scr > 75th percentile) and ELBW neonates without raised creatinemia (peak Scr < 75th percentile) are provided in Table 1. Covariates that were significantly different in the monovariate analysis were entered in a multiple regression model following log transformation when appropriated. GA and Apgar scores at 10 min were independent covariates associated with a higher peak Scr (Radj 0.32). To further explore the interaction between different covariates, a logistic regression model was applied with peak Scr (either > or < 75th percentile) as dichotomous (37/114 cases variable. Lower GA (OR 0.45; 95% CI 0.31–0.64) and longer duration of ventilation (OR 1.03; 95% CI 1.01–1.06) were identified as independent covariates of raised creatinemia in ELBW neonates.
Postnatal trends (median + 95% CI) in creatinemia in extreme low birth weight infants with a peak creatinemia either above (n = 37) or below (n = 114) the 75th percentile (i.e., 112.3 μmol/l). Creatinemia remains elevated in the raised peak creatinemia subgroup up to day 28 without statistically significant differences on day 42
When the dichotomous (15/136) approach for raised creatinemia was at the 90th percentile, creatinemia in cases (n = 15) remained significantly higher throughout the postnatal time interval evaluated, including day 42 (all at least p < 0.05). In a regression model, GA remained the only independent covariate of raised creatinemia (>P90) (Radj 0.13, OR 0.36; 95% CI 0.22–0.59).
Discussion
Based on a cohort of 151 ELBW neonates, we described postnatal trends in Scr (initial rise and subsequent decrease) and its covariates (GA and disease severity, reflected by the Apgar score or duration of ventilation) in the first 6 weeks of postnatal life. These covariates were assessed based on a dynamic definition of raised creatinemia, i.e., the 75th percentile of peak Scr (112.3 μmol/l). Finally, we evaluated to what extent and until when (<day 3) neonatal Scr still reflects maternal Scr.
It is well known that early postnatal life is characterized by a physiological rise in Scr. This paper provides weight based references values in ELBW (i.e., < 1,000 g) infants while Guignard et al. described this phenomenon in neonates down to 1,000 g and documented that this postnatal rise is most pronounced in neonates with the lowest birth weight. We confirm this trend in ELBW neonates since a higher initial peak and a subsequent postnatal delayed normalization has been documented (Fig. 1) [4]. Such a postnatal rise and subsequent progressive decrease in Scr is in part due to differences in GFR, in part due to differences in the extent and duration of passive tubular back leak of creatinine [12]. The fact that the increase in creatinemia in ELBW is more pronounced and the subsequent normalization more delayed, is likely a reflection of both phenomena [12]. This postnatal decrease has been further described throughout infancy by Boer et al. as the ‘broken stick’ model with a stabilization in creatinemia between the second and the seventh month of postnatal life in healthy term neonates [14].
Median, P25–75, P90, and range values of serum creatinine for the consecutive time intervals in the first 6 weeks of postnatal age in ELBW infants are provided (Table 2). There are, however, two methodological constraints to be mentioned before these percentiles can be used. Firstly, median P50, P25–75, and 90th percentile instead of mean + 2 SD have been used since not all results displayed normal distribution. Secondly, all creatinine assessments were based on the Jaffe reaction technique. In essence, this technique quantifies creatinine based on a colorimetric assessment but this analysis is also influenced by endogenous (e.g., bilirubin) or exogenous (e.g., penicillin) compounds frequently present in the serum of a newborn. More recently, an enzymatic technique to quantify creatinine has been introduced but specific conversion factors between both techniques need validation in ELBW neonates. In this cohort, cases treated with raised hyperbilirubinemia (> 85.5 μmol/l), reflected by the use of phototherapy (n = 122) had no higher creatinemia compared to cases without raised hyperbilirubinemia throughout the first 10 days of postnatal life. Consequently, this was not a significant indicator (Table 1) of raised creatinemia. We hypothesize that this absence of difference is likely due to the much lower threshold to initiate phototherapy compared to more mature neonates.
Indicators of both immaturity (i.e., GA) and disease severity (Apgar score or duration of respiratory support) were identified as important covariates of raised creatinemia (Scr > 75th percentile, i.e., 112.3 μmol/l) within this cohort of 151 ELBW neonates. When limited to cases with Scr > 90th percentile, GA was the only independent covariate. The dominant impact of GA, likely reflecting maturation instead of weight on postnatal creatinemia trends, confirms earlier GA-focused reports [2, 11, 13, 15–18]. Consequently, and based on the current analysis in ELBW neonates, we confirm that gestational and postnatal age based reference charts to assess renal function in ELBW neonates like Gallini (≤32 weeks GA) [16], Thayyil et al. (≤28 weeks GA) [17], or Iacobelli et al. (<32 weeks GA) [18] better reflect the maturational trends compared to a weight and postnatal age-based reference charts [4].
Although the number of papers that focus on risk factors of raised creatinemia in ELBW neonates is still limited, we anticipated ibuprofen also to be an independent covariate of raised creatinemia [18–20]. When comparing Scr observations in ELBW neonates treated with ibuprofen (n = 77/150) to those not treated, we document a negative effect of ibuprofen exposure on peak Scr (peak Scr > P75 = 25/37 ibuprofen exposed, Table 1). Serum creatinine remained significantly elevated in exposed ELBW neonates up to the fourth week of postnatal life (day 21: 61 vs. 58 μmol/l, p < 0.05) (Fig. 4). We hereby confirm the findings of Vieux et al. that the negative impact of ibuprofen on creatinine clearance extends beyond the days of ibuprofen exposure [19].
Serum creatinemia (median and 95% CI) observations as collected in ELBW neonates exposed (n = 77) or not exposed (n = 73) to ibuprofen in early neonatal life. In ELBW neonates exposed to ibuprofen, creatinemia remained significantly (monovariate, at least p < 0.05) higher up to the fourth week of postnatal life
However, when all risk factors were entered in multiple or logistic regression models, immaturity turned out to be the most predominant covariate of creatinemia and ibuprofen was no longer an independent covariate. At least this does suggest that ibuprofen serves as an indicator of impaired renal clearance throughout neonatal life (<day 28) even after exposure has been stopped, but that the delayed normalization in Scr or creatinine clearance more likely reflects immaturity (lower gestational age) and (respiratory) morbidity instead of an persistent renal ibuprofen induced toxic effect [19, 20].
The introduction of a descriptive cut-off value (Scr >75th percentile for a given cohort) for raised creatinemia is open to criticism. We are aware that inulin clearance, creatinine clearance, or aminoglycoside clearance very likely are better indicators of glomerular filtration rate in neonatal life [3, 11–13]. However, in the clinical setting, Scr is a readily accessible, available method to estimate renal function in preterm infants, and therefore allows risk stratification in a clinical setting.
It is hereby intriguing that raised peak Scr (peak Scr >75th percentile) resulted in raised Scr up to day 28, but no longer on day 42 (Fig. 3) similar to that we described using interindividual variability of amikacin clearance as an indicator of GFR in neonates up to 28 days of postnatal life [5, 13]. The concept of raised Scr in ELBW infants during neonatal life with a trend towards normalization in the first year of life and a subsequent deterioration in a subgroup (9/20) during childhood (mean 7.5 year) has been described by Abitbol et al. in a preselected cohort [21]. It is therefore possible that the progressive normalization after the peak in the ELBW neonates with initial raised creatinemia only reflects transient renal dysfunction. However, the neonate has potentially reached a new steady state regarding its creatinemia through hyperfiltration or other adaptive processes [6–10]. Since preterm birth itself has been associated with a low glomerular number, increasing the risk of glomerular sclerosis and systemic hypertension later in life, a focused assessment of final renal outcome in the subgroup with ‘transient raised creatinemia’ is warranted [6–10].
It has been repeatedly mentioned that neonatal Scr in the first days of life mainly reflects maternal Scr, although we are unaware of focused assessment in ELBW neonates. Based on 80 maternal-ELBW pairs, we documented that neonatal Scr mainly relates to maternal Scr on day 1, with a subsequent progressive weakening link, as also reflected in the Bland–Altman plots (Fig. 2 a/b). We hereby reconfirm that GFR at birth should be estimated based on GA and disease characteristics (e.g., Apgar score, asphyxia) to guide medical (e.g., fluid, drug) treatment, and that therapeutic drug monitoring should be considered in the very first days of life when (side)effects of a given compound (e.g., aminoglycosides) relate to renal elimination capacity [1–5].
In conclusion, we described postnatal serum creatinine trends and its covariates in ELBW neonates. Raised creatinemia in ELBW neonates (defined by peak Scr > P75, i.e., 112.3 μmol/l) was associated with immaturity (lower GA) and disease severity (duration of ventilation period, Apgar score). Peak Scr was a good predictor for subsequent Scr trends up to 28 days of postnatal life.
References
Cataldi L, Leone R, Moretti U, De Mitri B, Fanos V, Ruggeri L, Sabatino G, Torcasio F, Zanardo V, Attardo G, Riccobene F, Martano C, Benini D, Cuzzolin L (2005) Potential risk factors for development of acute renal failure in preterm newborn infants: a case control study. Arch Dis Child Fetal Neonatal Ed 90:F514–F519
Cuzzolin L, Fanos V, Pinna B, de Marzio M, Perin M, Tramontozzi P, Tonetto P, Cataldi L (2006) Postnatal renal function in preterm newborns: a role of diseases, drugs and therapeutic interventions. Pediatr Nephrol 21:931–938
Drukker A, Guignard JP (2002) Renal aspects of the term and preterm infant: a selective update. Curr Opin Pediatr 14:175–182
Bueva A, Guignard JP (1994) Renal function in preterm neonates. Pediatr Res 36:572–577
Van den Anker JN, Allegaert K (2009) Individualized dosing of aminoglycosides in neonates: mission accomplished or work in progress? Eur J Clin Pharmacol 65:1159–1160
Keijzer-Veen MG, Dülger A, Dekker FW, Nauta J, van der Heijden BJ (2010) Very preterm birth is a risk factor for increased systolic blood pressure at a young adult age. Pediatr Nephrol 25:509–516
Keijzer-Veen M, Devos AS, Meradji M, Dekker FW, Nauta J, van der Heijden BJ (2010) Reduced renal length and volume 20 years after very preterm birth. Pediatr Nephrol 25:499–507
Bachetta J, Harambat J, Dubourg L, Guy B, Liutkus A, Canterino I, Kassai B, Putet G, Cochat P (2009) Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int 76:445–452
Zaffanello M, Brugnara M, Bruno C, Franchi B, Talamini G, Guidi G, Cataldi L, Biban P, Mella R, Fanos V (2010) Renal function and volume of infants born with a very low birth-weight: a preliminary cross-sectional study. Acta Paediatr 99:1192–1198
Puddu M, Fanos V, Podda F, Zaffanello M (2009) The kidney for prenatal to adult life: perinatal programming and reduction of number of nephrons during development. Am J Nephrol 30:162–170
Vieux R, Hascoet JM, Merdariu D, Fresson J, Guillemin F (2010) Glomerular filtration rate reference values in very preterm children. Pediatrics 125:e1186–e1192
Guignard JP, Drukker A (1999) Why do newborn infants have a high plasma creatinine? Pediatrics 103:e49
Allegaert K, Anderson BJ, van den Anker JN, Vanhaesebrouck S, de Zegher F (2007) Renal drug clearance in preterm neonates: relation to prenatal growth. Ther Drug Monit 29:284–291
Boer DP, de Rijke YB, Hop WC, Cransberg K, Dorresteijn EM (2010) Reference values for serum creatinine in children younger than 1 year of age. Pediatr Nephrol 25:2107–2113
Rudd PT, Hughes EA, Placzek MM, Hodes DT (1983) Reference ranges for plasma creatinine during the first month of life (1983). Arch Dis Child 58:212–215
Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G (2000) Progression of renal function in preterm neonates with gestational age ≤ 32 weeks. Pediatr Nephrol 15:119–124
Thayyil S, Sheik S, Kempley ST, Sinha A (2008) A gestation- and postnatal age-based reference chart for assessing renal function in extremely premature infants. J Perinatol 28:226–229
Iacobelli S, Bonsante F, Ferdinus C, Labenne M, Gouyon JB (2009) Factors affecting postnatal changes in serum creatinine in preterm infants with gestational age <32 weeks. J Perinatol 29:232–236
Vieux R, Desandes R, Boubred F, Semama D, Guillemin F, Buchweiller MC, Fesson J, Hascoet JM (2010) Ibuprofen in very preterm infants impairs renal function for the first month of life. Pediatr Nephrol 25:267–274
Allegaert K, Cossey V, Debeer A, Langhendries JP, Van Overmeire B, de Hoon J, Devlieger H (2005) The impact of ibuprofen on renal clearance in preterm infants in independent of the gestational age. Pediatr Nephrol 20:740–743
Abitbol CL, Bauer CR, Montané B, Chandar J, Duara S, Zilleruelo G (2003) Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol 18:887–893
Acknowledgements
Elena Levtchenko and Karel Allegaert are supported by the Fund for Scientific Research, Flanders (Belgium) (F.W.O. Vlaanderen) by a Fundamental Clinical Investigatorship (1801110 N and 1800209 N).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
George, I., Mekahli, D., Rayyan, M. et al. Postnatal trends in creatinemia and its covariates in extremely low birth weight (ELBW) neonates. Pediatr Nephrol 26, 1843–1849 (2011). https://doi.org/10.1007/s00467-011-1883-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1883-0