Abstract
Data on conservative treatment in children with urolithiasis are limited. The aim of the study was to determine the metabolic etiology and results of conservative treatment in children with urolithiasis. We evaluated the clinical presentation and metabolic features of 112 children with urolithiasis. The mean age at diagnosis of urolithiasis was 3.9 (range 0.1–18) years, and follow-up duration was 16.7 (range 1–36) months. The most common presenting symptoms were flank or abdominal pain and restlessness (25%). Urine analysis revealed metabolic abnormalities in 92% of cases, including hypocitraturia (42%), hyperoxaluria (32.1%), hypercalcuria (25%), hyperuricosuria (9.8%), and cystinuria (2.7%). Patients who had metabolic risk factors were treated according to underlying metabolic abnormalities. About half of these patients were stone free or stones were diminished in size. These results showed that early recognition and treatment of urinary metabolic abnormalities will reduce the number of invasive procedures and renal damage in children with urolithiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is a significant health problem and very common in some parts of the world. Most recent studies report that the incidence has increased significantly in both adults and children [1, 2]. Incidence in Turkish school children was reported to be 0.8% [3]. Urolithiasis can cause deterioration of renal function and permanent damage to the kidney. It has been reported that 4–8% of all cases of chronic renal failure in Turkey have been secondary to urinary-stone disease [4, 5]. Urolithiasis has multifactorial etiologies. Apart from anatomical defects and infections, urolithiasis is more often associated with underlying metabolic abnormalities, which have been reported in 40–84% of children [6–8]. Determining specific etiology is very important to appropriate treatment. Medical treatment is aimed at protecting the patient from further growth of existing stones and from the development of new stones, thus decreasing morbidity and the need for surgical intervention. Hence, under these circumstances, medical treatment means prevention. To achieve this goal, it is important for one to obtain stone analysis, if feasible, and urine chemistry analysis as early as possible. In this prospective study, we investigated clinical features, metabolic risk factors, and medical treatment results in children with urolithiasis.
Patients and methods
The study enrolled 112 children with urolithiasis diagnosed and followed in the department of pediatric nephrology between May 2007 and April 2010. Urolithiasis was diagnosed using ultrasonography (US) by the two radiologists. In such cases, color Doppler US was used, as the wall of blood vessels can mimic stones appearing as echogenic foci. However, US or color Doppler US are not as sensitive as computed tomography (CT) for detecting small stones or stones in the ureter. Therefore, if stones were strongly suspected, the diagnosis was confirmed by CT without the use of contrast agents. Urinary calculi ≤3 mm were defined as microlithiasis and urinary calculi >3 mm as urolithiasis [9]. Voiding cystourethrography (VCUG) and intravenous pyelography (IVP) are not done routinely in patients with urolithiasis. VCUG was done in patients with recurrent urinary tract infection (UTI) and/or hydronephrosis. Patients with structural urinary tract obstruction were not included in the study.
Blood serum and urine analysis were performed when the patients were first seen by the clinician. Urine cultures were sent for bacteriological examination if there were clinical and laboratory findings of UTI. In patients with UTI, metabolic evaluation was performed after the UTI was treated. Twenty-four-hour urine collection was obtained from all patients, and samples were placed in an acidified container 0.2 ml 6 M hydrochloride (HCl) for calcium, in alkaline media for uric acid, and in neutral media for oxalate, citrate, and cystine testing; samples were sent the laboratory without delay. Twenty-four-hour urine analysis according to the normal values was taken into consideration. Hypercalcuria, hyperoxaluria, hyperuricosuria, and cystinuria was diagnosed if the amounts of those compounds in the urine >4 mg/kg per 24 h, 0.5 mmol (45 mg)/1.73 m2 per 24 h, 815 mg/1.73 m2 per 24 h, and 75 mg/1.73 m2 per 24 h, respectively. Hypocitraturia was defined as citrate excretion >320 mg/1.73 m2 per 24 h [8]. Stone samples obtained by spontaneous passage, or open operation in six children, were sent to the Institute of Mineral Inspection and Research Laboratory for analysis.
Therapeutic interventions
Fluid intake (>2.5 L/m2 day) was advised to all patients. Patients with metabolic risk factors were treated according to underlying metabolic abnormalities. In children with hypercalciuria, dietary sodium restriction along with high-potassium and potassium citrate solution (potassium citrate 100 mg, sodium citrate 100 mg in each 1,000 ml) or potassium citrate pill (Urocit-K®; contains potassium citrate 540 mg in each pill) was used. Potassium citrate solution contains approximately 2 mEq/ml potassium. Starting dosage of solution is 2 ml/kg body weight divided into three doses a day. Chlorothiazide (15–25 mg/kg day) was offered to hypercalciuric patients resistant to dietary modifications and potassium citrate. In children with hyperoxaluria, foods rich in oxalate and ascorbic acid were restricted. Pyridoxine (10–20 mg/kg day) was also administered. Potassium citrate was used in patients with hyperoxaluria who were unresponsive to pyridoxine and dietary modifications. In children with hypocitraturia only, potassium citrate was administered. In children with hyperuricosuria, fluid intake together with alkalinization of the urine to a pH value >7 with an appropriate agent (potassium citrate or sodium bicarbonate) was administered. In children with cystinuria, dietary sodium and protein restriction and urinary alkalinization with potassium citrate to maintain a urinary pH >7 were recommended. Tiopronin (Thiola ® 15 mg/kg day) was used in patients with hypercystinuria who were unresponsive to potassium citrate and dietary modifications [10].
Follow-up
All patients were followed up every 4–8 weeks for stone status. Serial US was used at each visit. Urine dipstick tests and microscopic examinations were performed at every control. Both urine pH and density were evaluated to assess the effect of administration of alkali or to check the patient’s compliance regarding sufficient fluid intake. Potassium citrate was stopped after 6 months of the patient being stone free. Patients in whom stone size increased and urinary obstruction developed were sent to a pediatric urologist.
Statistical analysis
Descriptive statistics were performed as statistical analysis using the computer software SPSS version 11.5.
Results
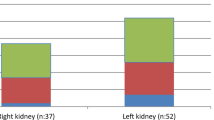
In this study, the patient group consisted of 62 male and 50 female patients (ratio 1.24:1). Mean age at diagnosis was 3.9 (range 0.1–18) years and follow-up duration 16.7 (range 1–36) months. On admission, 31 children (27.7%) were <1 year, 46 (41.1%) 1–5 years, 22 (19.6%) 5–10 years, and 13 (11.6%) >10 years. The most common presenting symptoms on admission were abdominal and/or flank pain and restlessness (25%). Presenting symptoms and features of patients with urolithiasis are shown in Table 1. Thirty-seven children (33%) had a history of recurrent UTI. A positive family history for urolithiasis was determined in 62 children (55.4%). Stones were located in the kidney in 105 of 112 children (93.8%), in the ureter in two (1.8%), and in both the kidney and the ureter in five (4.4%). Twenty-six patients (23.2%) had multiple stones with bilateral localization. Stones measuring ≤ 3 mm (microlithiasis) were found in 28 (25%) children, whereas 87 (75%) children had stones >3 mm. Vesicoureteral reflux was detected in 12 children. Reflux was graded as I in ten children and III in two children. Serum calcium levels were >10.8 mg/dl in five children. Patients with hypercalcemia had normal parathormone levels.
Urine evaluation revealed that the most common metabolic abnormalities were hypocitraturia in 47 (42%) children, followed by hyperoxaluria in 36 (32.1%), hypercalcuria in 28 (25%), hyperuricosuria in 11 (9.8%), and cystinuria in three (2.7%). Only nine (8%) children were normal for all parameters. Although hypocitraturia was the most common metabolic abnormality for all ages, hypercalcuria (41.7%) was the most common in children >10 years. Patients also had multiple urine risk factors (Table 2). Stone analysis was available for six children. Stone size was between 3 and 15 mm. Four stones were whewellite and/or weddellite, one was cystine, and one was struvite.
Patients with metabolic risk factors were treated according to underlying metabolic abnormalities. One hundred and four children were followed up with only conservative treatment (pharmacological and/or nonpharmacological). At the time of their last outpatient visit, stone condition of these patients was evaluation. Fifty-nine (52.7%) were stone free or the stone was diminished in size; 43 (38.4%) showed no alteration in stone size; whereas stone size increased in ten (8.9%). When patients who were stone free or in whom stone size was diminished were compared with the other patients, it was determined that the initial size of stones was lower in children who became stone free or whose stone size diminished (mean size 5.1 ± 2.3 mm compared with 6.4 ± 3.3 mm, p < 0.05). Furthermore, stone-free patients or those with diminished stone size were determined to have hypocitraturia 42.4% as the most frequent metabolic abnormality. However, hyperoxaluria 50% was the most frequent metabolic abnormality in patients whose stone grew during treatment. Because of urinary obstruction, extracorporeal shock-wave lithotripsy (ESWL) was performed in one patient and open surgery in seven. Treatment results are shown in Table 3.
Discussion
Determining the etiology in urolithiasis cases plays a key role in planning successful treatment and preventing recurrence. Over recent decades, the etiology of urolithiasis in children has shifted from predominantly infectious to metabolic causes [8]. Many studies have reported a male predominance in childhood urolithiasis, with the ratio varying from 1.2:1 to 4:1 depending on the series [7, 11, 12]. In our study, urolithiasis was detected more commonly in males, as shown in those studies. Recent studies from various countries reported a mean age of 4.2–8.2 years for urolithiasis cases [7, 11–14]. We found a lower mean age of 3.9 years in our study; 68.8% of children were <5 years of age at admission. Children with nonspecific symptoms were evaluated by renal US, and this may have led to the early diagnosis of urolithiasis in our study. In the literature, the ratio of family history varies from 11.8% to 21.9% [12, 13, 15, 16]. A positive family history of urolithiasis was present in half of our patients. The rate of intermarriage in our region is very high, which may be related to the genetic tendency toward urolithiasis in our study group. The majority of urolithiasis identified in our study was located in the upper urinary tract, which is consistent with the results of many recent studies [7, 11, 12, 17]. The presenting signs and symptoms of pediatric urolithiasis are different from those in adults. The most common findings in children are flank or abdominal pain with or without hematuria Similar to our results, flank or abdominal pain, hematuria, and restlessness were reported as the most common symptoms in previous studies [7, 8, 11, 17, 18].
Urinary metabolic abnormality was present in 92% of cases, the most frequent being hypocitraturia. Other abnormalities were hyperoxaluria, hypercalcuria, hyperuricosuria, and cystinuria. In a recent series from Turkey, Tefekli et al. [19] found hypocitraturia in 60.6% of children with urolithiasis, being the most prevalent metabolic risk factor in that group. In other recent series in children, VanDervoort et al. [2] found that hypocitraturia is the most frequent urine metabolic abnormality in children. However, some reports from western Turkey stress that hypercalcuria was the predominant risk factors in children with urolithiasis [11]. Together with hypocitraturia, hypercalcuria and hyperoxaluria are important risk factors in our region, whereas in another Turkish city, hypercalcuria and hyperuricosuria were the most common metabolic abnormalities [17]. As some recent studies have reported, the probability of hypocitraturia was higher in the younger children with urolithiasis [20]. It seems that the frequency of urinary metabolic abnormalities change with age; however, environment and diet are also important frequency risk factors [21]. Thus, an underlying metabolic risk factor has been identified in many cases of urolithiasis, justifying detailed metabolic evaluation of each child who has once had urinary stone disease.
After the metabolic risk factor was determined, 92.8% of our patients were followed up with only conservative treatment according to underlying metabolic abnormalities. Half of these patients were stone free or had diminished stone size during follow-up. Only eight patients required ESWL or open surgery because of urinary obstruction. The most prevalent metabolic risk factor was hypocitraturia in stone-free patients or those with diminished stone size, whereas the most prevalent metabolic risk factor was hyperoxaluria in patients with increased stone size. Some studies reported conservative treatment for children with hypocitraturia, which was, however, not successful for children with hyperoxaluria because most of the excreted oxalate was endogenously produced [22, 23].One limitation of our study is that we did not determine whether hyperoxaluria was genetic or absorptive.
In conclusion, identifying specific predisposing metabolic factors in each patient is important for developing the most effective therapeutic regimen. The appropriate investigations of a child presenting with signs and symptoms of urolithiasis will allow earlier recognition of the problem. Preventing renal damage and disease recurrence may be possible with appropriate treatment of the urinary metabolic abnormality in children with urolithiasis.
References
Goldfarb DS (2003) Increasing prevalence of kidney stones in the United States. Kidney Int 63:1951–1952
VanDervoot K, Wiesen J, Frank R, Vento S, Crosby V, Chadra M, Trachtman H (2007) Urolithiasis in pediatric patients: a single center study of incidence, clinical presentation and outcome. J Urol 177:2300–2305
Remzi D, Çakmak F, Erkan I (1980) A study on urolithiasis incidence in school age children. J Urol 123:608–610
Sirin A, Emre S, Alpay H, Nayir A, Bilge I, Tanman F (1995) Etiology of chronic renal failure in Turkish children. Pediatr Nephrol 9:549–552
Turkey National Hemodialysis (2004) Transplantation and Nephrology Registry Report of Turkey 2003. Istanbul. Available at: https://doi.org/www.tsn.org.tr/registry/Registry_2003_Tr_Eng.pdf
Erbagci A, Erbagci AB, Yılmaz M, Yagci F, Trakcioglu M, Yurtseven C, Koyluoglu O, Sarica K (2003) Pedaitric urolithiasis evaluation of risk factors in 95 children. Scand J Urol Nephrol 37:129–133
Bak M, Ural R, Agin H, Serdaroglu E, Calkavur S (2009) The metabolic etiology of urolithiasis in Turkish children. Int Urol Nephrol 41:453–460
Cameron MA, Sakhaee K, Moe OW (2005) Nephrolithiasis in children. Pediatr Nephrol 20:1587–1592
Polito C, Cioce F, La Manna A, Maiello R, Di Torro R (1999) Renal calyceal microlithiasis: clinical presentation may precede sonographic evidence. Clin Pediatr (Phila) 38:521–524
Alon US (2009) Medical treatment of pediatric urolithiasis. Pediatr Nephrol 24:2129–2135
Alpay H, Ozen A, Gokce I, Biyikli N (2009) Clinical and metabolic features of urolithiasis and microlithiasis in children. Pediatr Nephrol 24:2203–2209
Ozkutan BH, Küçükaydın M, Gündüz Z, Kabaklioğlu M, Okur H, Turan C (2000) Urolithiasis in childhood. Pediatr Surg Int 16:60–63
Oner A, Demircin G, İpekcioğlu H, Bulbul M, Ecin N (1997) Etiological and clinical patterns of urolithiasis in Turkish children. Eur Urol 31:453–458
Perrone HC, Santos DR, Santos MV, Pinherio ME, Toporovski J, Ramos OL, Schor N (1992) Urolithiasis in childhood: metabolic evaluation. Pediatr Nephrol 6:54–56
Gerhart JP, Herzberg GZ, Jeffs RD (1991) Chilhood urolithiasis: experience and advances. Paediatrics 87:445–450
Hari P, Bagga A, Vasudev V, Singh M, Srivastava RN (1995) Aetiology of nephrolithiasis in north Indian children. Pediatr Nephrol 9:474–475
Dursun I, Poyrazoglu MH, Düşünsel R, Gündüz Z, Gürgöze MK, Demirci D, Küçükaydın M (2008) Pediatric urolithiasis: an 8-year experience of single center. Int Urol Nephrol 40:3–9
Hulton SA (2001) Evaluation of urinary tract calculi in children. Arch Dis Child 84:320–323
Tefekli A, Esen T, Ziylan O, Erol B, Armağan A, Ander H, Akıncı M (2000) Metabolic risk factors in pediatric and adult calcium oxalate urinary stone formers: is there any difference? Urol Int 459:1–10
Kalorin CM, Zabinski A, Okpareke I, White M, Kogan BA (2009) Pediatric urinary stones disease-does age matter? J Urol 181:2267–2271
Spivacow FR, Negri AL, del Valle EE, Calvino I, Zanchetta JR (2009) Clinical and metabolic risk factor evaluation in young adults with kidney stones. Int Urol Nephrol 42:471–475
Tekin A, Tekgül S, Atsu N, Bakkaloglu M, Kendi S (2002) Oral potassium citrate treatment for idiopathic hypocitraturia in children with calcium urolithiasis. J Urol 168:2572–2574
Sarica K (2006) Pediatric urolithiasis: etiology, specific pathogenesis and medical treatment. Urol Res 34:96–101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gürgöze, M.K., Sarı, M.Y. Results of medical treatment and metabolic risk factors in children with urolithiasis. Pediatr Nephrol 26, 933–937 (2011). https://doi.org/10.1007/s00467-011-1803-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1803-3