Abstract
The aim of this study was to establish age appropriate reference intervals for calcium (Ca), phosphorus (P) and total protein (UTP) in random urine samples. All analytes were measured using the Roche MODULAR P analyzer and normalized to creatinine (Cr). Our study cohort consisted of 674 boys and 728 girls between 7 and 17 years old (y.o.), which allowed us to determine the central 95% reference intervals with 90% confidence intervals by non-parametric analysis partitioned by both gender and 2-year age intervals for each analyte [i.e. boys in age group 7–9 years (7–9 boys); girls in age group 7–9 years (7–9 girls), etc.]. Results for the upper limits of the central 95% reference interval were: for Ca/Cr, 0.27 (16,17 y.o.) to 0.46 mg/mg (7–9 y.o.) for the girls and 0.26 (16,17 y.o.) to 0.43 mg/mg (7–9 y.o.) for the boys; for P/Cr, 0.85 (16,17 y.o.) to 1.44 mg/mg (7–9 y.o.) for the girls and 0.87 (16,17 y.o.) to 1.68 mg/mg (7–9 y.o.) for the boys; for UTP/Cr, 0.30 (7–9 y.o.) to 0.34 mg/mg (10–12 y.o.) for the girls and 0.19 (16,17, y.o.) to 0.26 mg/mg (13–15 y.o.) for the boys. Upper reference limits decreased with increasing age, and age was a statistically significant variable for all analytes. Eight separate age- and gender-specific reference intervals are proposed per analyte.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric reference intervals are often difficult to establish. Obtaining sufficient blood or urine samples from healthy children can be a challenge, and physicians are often faced with the lack of pediatric reference intervals for many clinically important analytes. Pediatric reference intervals for urinary analytes such as calcium (Ca) and phosphorus (P) are particularly useful in evaluating disorders of mineral metabolism. Many studies have addressed calcium metabolism and urine calcium reference intervals in children [1–13]. The current gold standard for diagnosing hypercalciuria is >4 mg/kg/day using a 24-h urine collection [8, 14]. However, accurate and complete 24-h urine collections are difficult to obtain, and spot urine calcium/creatinine (Ca/Cr) ratios have become more commonly used in clinical practice. Spot Ca/Cr ratios of 0.21 (mg/mg) have been suggested as the upper normal limit for diagnosing hypercalciuria [15]. However, urinary calcium reference intervals have been shown to vary with age and geographical distribution [1, 3, 9, 10, 16]. Urinary phosphorus excretion assessment is important in a number of clinical scenarios, such as metabolic acidosis, renal tubular disorders, vitamin D intoxication, hyperthyroidism and urothialisis, yet data on urinary phosphorus reference intervals are quite limited [1, 4, 9, 10]. Elevated concentrations of urine calcium, phosphorus and total protein can also be associated with kidney disease. Both renal calculi and renal disease are relatively rare pediatric conditions, but both are increasing in incidence. The causative agents responsible for the rising incidence in kidney disease and stone formation are unclear, although it is suspected that diet plays an important role. Therefore, pediatric reference intervals for calcium, phosphorus and total protein have become increasingly useful, if not necessary.
The aim of this study was to establish age-appropriate reference intervals for calcium, phosphorus and total protein in random urine samples, to aid in diagnosis of mineral metabolism disorders and kidney disease, using a large cohort of over 1400 healthy children aged 7–17 years.
Materials and methods
A total of 1402 healthy children were enrolled in this study, which took place in Salt Lake City over a study interval of 5 years. Healthy children were recruited from the local community using magazine ads and flyers posted in local schools and libraries. Non-fasting urine samples were collected from each child after parental permission had been obtained. All studies using samples from human subjects were approved by the University of Utah Institutional Review Board. All urine samples were tested for hemolysis, presence of nitrites and leukocytes by dipstick analysis (Multistix 5; Bayer Healthcare Diagnostics Division, Newbury, UK). Samples that tested positive for nitrites or ++ (moderate) for either leukocytes and/or hemolysis were eliminated from further analysis. Therefore, reference intervals were determined using 1263 urinary samples obtained from healthy children, 660 boys and 603 girls aged 7–17 years. All urine analytes were measured using a Modular P analyzer (Roche Diagnostics, Indianapolis, IN) as follows: calcium by the o-cresolphthalein spectrophotometric method, phosphorus by an ammonium molybdate method, total protein by a benzathonium method and creatinine by a modified Jaffe reaction. Because all samples were random urine specimens, all analytes were normalized for creatinine and reported as analyte-to-creatinine ratios (mg/mg). Reference intervals were partitioned by both gender and 2-year age intervals. Non-parametric analysis using EP Evaluator release 8 software (David G. Rhoads Assoc, Kennett Square, PA) was conducted to determine the central 95% reference intervals with 90% confidence intervals (CIs). Samples deemed to be outliers based on the Dixon/Reed/CLSI rule were excluded. Briefly, the range of the values = R, and the absolute difference between the extreme value and the next closest value = D. If the ratio of D/R exceeds 1/3, then the result is considered to be an outlier and excluded from the reference intervals study [17]. A Wilcoxon signed-rank test performed using the R Project (Comprehensive R Network Archive) was also utilized to assess gender and age differences.
Results
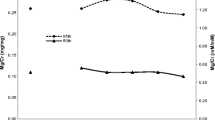
Non-parametric analysis and the central 95% reference intervals with 90% CIs for the three analytes based on 2-year age intervals and gender are summarized in Table 1. The central 95% upper limit of the reference intervals for Ca/Cr ranged from 0.27 [age group 16 and 17 years; hereafter 16, 17 years old (y.o.)] to 0.46 mg/mg (7–9 y.o.) for the girls and from 0.26 (16–17 y.o.) to 0.43 mg/mg (7–9 y.o.) for the boys. For calcium excretion, trends included decreases in analyte concentration with age for both genders and generally lower analyte concentrations for boys relative to girls in the same age group. The 7- to 9-year age group, both genders, had skewed results, with a significant tail at the upper reference interval limit that was slightly more pronounced in girls. Because of this skewed distribution of results in the youngest age group and the decline in analyte concentration observed with age, the 90% CI of this age group did not overlap with at least one of the older age groups, for both genders. In addition, the Wilcoxon signed rank test found that age differences were significant (p < 0.0001) but gender was not (p = 0.08).
For phosphorus, similar trends were observed. The upper limits of P/Cr reference intervals ranged from 0.85 (16, 17 y.o.) to 1.44 mg/mg (7–9 y.o.) for the girls and from 0.87 (16, 17 y.o.) to 1.68 mg/mg (7–9 y.o.) for boys. Phosphorus concentrations declined with increasing age, but in this case, girls had slightly lower ratios than boys. Again because of the age-dependent analyte concentration decrease, the 90% CI of the 7- to 9-year age group did not overlap with at least one of the older age groups, for both genders. Although the 90% CI for the central 95% reference interval did overlap between genders for all age groups, there were differences between boys and girls. For example, the upper reference limit of the 13- to 15-year girls age group, 0.93 mg/mg P/Cr, is not encompassed by the boys 90% CI or by the combined boys and girls 90% CI for that age group (Table 1). Both age (p < 0.0001) and gender (p = 0.04) differences were significant for P/Cr.
The urine total protein (UTP) data were more complex. The upper limits for UTP/Cr ranged from 0.30 (7–9 y.o.) to 0.34 mg/mg (10–12 y.o.) for the girls and from 0.19 (16, 17 y.o.) to 0.26 mg/mg (13–15 y.o.) for boys. Age-based differences were observed, including a pronounced increase in concentration in the 13- to 15-year boys age group, followed by a decrease in the 16- to 17-year boys age group. Girls had higher UTP/Cr ratios than boys for all age groups. Age-based differences (p < 0.0001) were significant but gender was not, when analyzed by the Wilcoxon signed-rank test.
Discussion
Because we had a large number of samples, over 1200, we were able to calculate the central 95% reference interval with relatively small confidence intervals for each age and gender group. Although this is the preferred and appropriate approach for asymmetric distributions, it has been difficult to attain this type of analysis in previous studies due to the large numbers of samples required. The results of our study suggest that separate reference intervals that are both age- and gender-specific are warranted for Ca/Cr, P/Cr and UTP/Cr for children aged 7–17 years. Our statistical analysis demonstrated that age differences were significant for all three analytes tested; gender differences were not as pronounced. The results of the Wilcoxon signed-rank test for determining the significance of both gender and age for all three analytes suggested significant differences for four of the six paired group results. In addition, positively skewed results could be attributed to gender in some age groups for all analytes. We chose to establish separate gender-specific reference intervals partitioned by 2-year age groups for all three analytes, for a total of eight separate reference intervals per analyte.
For Ca/Cr, an inverse relationship between analyte concentration and age was observed, a finding that has been previously reported by several groups [1, 5, 9, 13]. We also found that age differences were statistically significant by the Wilcoxon signed-rank test (p < 0.0001). Gender differences were not significant for calcium excretion, but boys had lower concentrations than girls in the corresponding age group. Seifert and colleagues found gender to be significant [11], but other researchers have not [9, 13]. Reference limits for calcium for all age groups in our study were higher than those previously reported. Our values are most consistent with those reported in the Swedish study by Ejsborner, who found the 95% upper limit of Ca/Cr of the 7- to 10-year age group to be 0.42 mg/mg (n = 79) compared to our 0.43 mg/mg for boys and girls combined in the 7- to 9-year age group (n = 326) [5]. Ejsborner measured post-prandial Ca/Cr ratios and found higher values than those reported elsewhere. Interestingly, we did not collect fasting samples, and it has been demonstrated that meal intake can affect urinary calcium concentrations.
Our higher values for Ca/Cr may also be due to the worldwide variation in urinary calcium excretion that has been reported by various studies and reviewed by So and colleagues [16]. Several factors, including geography, diet, race, water source, exposure to sunlight and even season, have been implicated in affecting urinary calcium concentrations. Studies performed on Western pediatric populations have been associated with higher Ca/Cr values, most likely due to diet. Another possibility for the difference in results between studies may be the low number of subjects in each age and gender group and/or the use of parametric statistical analysis in previous studies.
Fewer studies have investigated urinary phosphorus excretion [1, 4, 9, 10]. In our study, phosphorus ratios progressively decreased with age, which has been demonstrated by others as well [1, 9, 10]. Unlike other studies, gender was also a significant variable in our data (p = 0.04 Wilcoxon signed-rank test). The central 95% reference upper limit was comparable to values found by Matos and colleagues [9]. The upper reference limit for P/Cr in the Matos study in the 10- to 14-year age group was 0.86 mg/mg as compared to our 97.5% upper reference limit of 0.93 mg/mg for girls and 1.13 mg/mg for boys.
Spot UTP/Cr ratios have been utilized for screening and monitoring chronic kidney disease in adults and children. The National Kidney Foundation guidelines recommend follow-up testing in children and adolescents with ratios >0.20 mg/mg, preferably on a first morning specimen. All but one of the upper reference limits in our study were above 0.20 mg/mg. Although method-dependent variation has been demonstrated, it is unlikely that this alone accounts for the difference in our results. In our study, only one sample per child, without any requirement for a first morning void, was collected. Therefore, the impact of children with orthostatic proteinuria on the results cannot be clearly assessed. The prevalence of orthostatic proteinuria has been estimated to be between 2 and 5% in adolescents. In addition, evidence of proteinuria in a single spot urine sample is relatively common, with an estimated prevalence of 5–15% [18]. Our results support the requirement of a first morning void sample if a cutoff of 0.20 mg/mg is utilized. Only age was a statistically significant variable for UTP/Cr by the Wilcoxon signed-rank test (p < 0.0001) in this study. Concentrations of urine total protein were higher in girls than in boys for all age groups, possibly because girls excrete less creatinine and, therefore, the ratio is increased in the younger age groups as compared to boys. However, as both genders go through puberty and the rapid growth associated with this period, the creatinine excretion changes due to body mass changes, thus affecting the UTP/Cr ratio. Increased UTP/Cr ratios in the 10- to 12-year girls age group and 13- to 15-year boys age group were observed. Further analysis is needed to determine the relationship of creatinine and urine total protein in relation to body mass.
Although our data are consistent with those published elsewhere, there were notable differences, such as a significant gender effect for phosphorus, the slightly higher levels of analyte observed for all age groups for Ca/Cr and a significant age effect for Ca/Cr, even for children above 7 years of age. Diet, which can affect two of the three analytes studied, Ca/Cr and P/Cr, to varying degrees may be one explanation for these discrepancies. Another possibility is that our data set is limited in ethnic representation. However, we believe that the different conclusions may also be attributed to the fact that our study, unlike previous studies, consisted of sufficient sample numbers for non-parametric statistical analysis of data partitioned by both gender and 2-year age intervals. The results of these analyses suggest that age- and gender-specific reference intervals are required for the analytes studied for children aged 7–17 years. Establishing pediatric reference intervals remains important, and we believe this work should aid in this endeavor.
References
Chen Y, Lee AJ, Chen CH, Chesney RW, Stapleton FB, Roy S 3rd (1994) Urinary mineral excretion among normal Taiwanese children. Pediatr Nephrol 8:36–39
Alconcher LF, Castro C, Quintana D, Abt N, Moran L, Gonzalez L, Cella M (1997) Urinary calcium excretion in healthy school children. Pediatr Nephrol 11:186–188
Ceran O, Akin M, Akturk Z, Ozkozaci T (2003) Normal urinary calcium/creatinine ratios in Turkish Children. Indian Pediatr 40:884–887
De Santo NG, Di Iorio B, Capasso G, Paduano C, Stamler R, Langman CB, Stamler J (1992) Population-based data on urinary excretion of calcium, magnesium, oxalate, phosphate and uric acid in children from Cimitile (Southern Italy). Pediatr Nephrol 6:149–157
Esbjorner E (1995) Urinary calcium excretion in Swedish children. Acta Paediatr 84:156–159
Koyun M, Guven AG, Filiz S, Akman S, Akbas H, Baysal YE, Dedeoglu N (2006) Screening for hypercalciuria in schoolchildren: what should be the criteria for diagnosis? Pediatr Nephrol 22:1297–1301
Kruse K, Kracht U, Kruse U (1984) Reference values for urinary calcium excretion and screening for hypercalciuria in children and adolescents. Eur J Pediatr 143:25–31
Manz F, Kehrt R, Lausen B, Merkel A (1999) Urinary calcium excretion in healthy children and adolescents. Pediatr Nephrol 13:894–899
Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard J (1997) Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr 131:252–257
Safarinejad MR (2003) Urinary mineral excretion in healthy Iranian children. Pediatr Nephrol 18:140–144
Seifert-McLean CM, Cromer BA, Mosher G, Mahan JD (1989) Urinary calcium excretion in healthy adolescents. J Adolesc Health Care 10:300–304
Sönmez F, Akcanal B, Altincik A, Yenisey C (2007) Urinary calcium excretion in healthy Turkish children. Int Urol Nephrol 39:917–922
Metz M (2006) Determining urinary calcium/creatinine cut-offs for the paediatric population using published data. Ann Clin Biochem 43:398–401
Moxey-Mims MM, Stapleton FB (1993) Hypercalciuria and nephrolcalcinois in children. Curr Opin Pediatr 5:186–190
Pak CY, Kaplan R, Bone H, Townsend J, Waters O (1975) A simple test for the diagnosis of absorptive, resorptive and renal hypercalciurias. N Engl J Med 292:497–500
So NP, Osorio AV, Simon SD, Alon US (2001) Normal urinary calcium/creatinine ratios in African-American and Caucasian children. Pediatr Nephrol 16:133–139
Horn PS, Feng L, Li Y, Pesce AJ (2001) Effect of outliers and nonhealthy individuals on reference interval estimation. Clin Chem 47:2137–2145
Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J (2000) Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105:1242–1249
Acknowledgments
Support for this study was provided by ARUP Institute for Clinical & Experimental Pathology. We gratefully acknowledge Andrew Wilson for assisting with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slev, P.R., Bunker, A.M., Owen, W.E. et al. Pediatric reference intervals for random urine calcium, phosphorus and total protein. Pediatr Nephrol 25, 1707–1710 (2010). https://doi.org/10.1007/s00467-010-1544-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1544-8