Abstract
The aim of work was to investigate whether serum and urinary neutrophil gelatinase-associated lipocalin (sNGAL and uNGAL, respectively) are potential biomarkers of early cyclosporine A (CsA) nephrotoxicity in steroid-dependent nephrotic children (SDNS). The study group (I) consisted of 19 children with SDNS aged 9.46 ± 5.52 years treated with CsA. The children were examined four times: at proteinuria relapse, prior to CsA treatment, then after 3, 6, and 12 months of CsA treatment. The control group (II) consisted of 18 healthy children aged 3–15 years. A commercial enzyme-linked immunosorbent assay method was used to measure NGAL concentration. The sNGAL level in SDNS children prior to the administration of CsA was similar to that in the healthy controls (p > 0.05), but it increased significantly during the course of treatment (p < 0.01). The uNGAL/creatinine (cr) ratio in SDNS patients was higher before the withdrawal of CsA therapy (p < 0.05), and was also increased at the consecutive examinations (p < 0.01). There was a positive correlation between both sNGAL and uNGAL levels and CsA serum level. However, based on the serum and urinary NGAL/cr receiver operating characteristic curve and area under the curve (AUC) analysis, it remains uncertain whether uNGAL is a good predictor of cyclosporine nephropathy. Both sNGAL and uNGAL concentrations increased during the course of CsA treatment. Further studies in larger groups of patients are therefore necessary to confirm our experimental data that increased NGAL levels may be a non-invasive marker for the early detection of tubulointerstitial damage in CsA nephrotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimal change nephrotic syndrome (MCNS) is the most common type of childhood nephrotic syndrome. Although most children respond well to steroid therapy, adjunctive therapy may be needed in some children with MCNS.
Therapy with the immunosuppressant cyclosporine A (CsA) has led to significant progress in the clinical outcome of steroid-dependent and steroid-resistant NS in children. However, the major complication of CsA in clinical use is nephrotoxicity, including renal haemodynamic abnormalities and, in particular, afferent arteriole vasoconstriction. Long-term treatment may lead to chronic nephropathy, characterized by renal ischaemic alterations with local inflammatory processes and fibrosis [1]. CsA-related renal toxicity may be detected in the form of a tubular injury [2]. Two mechanisms have been proposed: a direct tubular injury or vascular injury and ischemia. Therapy with CsA has been found to produce early sublethal injury in proximal tubule (PT) S3 segments, resulting in endoplasmic reticulum oedema [3] and increased excretion of urinary enzymes [4]. In current clinical practice, tubulointerstitial injury is typically diagnosed by renal biopsy or by the level of N-acethyl-beta-D-glucosaminindase (NAG) [5, 6]. However, the sensitivity and the specificity of NAG are not ideal, and its urinary level correlates with morphological changes only in the more advanced stages of the disease. In terms of CsA treatment, it would be clinically advantageous to find an early biomarker, i.e. at stages when urinary NAG levels are not yet elevated.
Neutrophil gelatinase-associated lipocalin (NGAL), also known as lipocalin-2, siderocalin, uterocalin and 24p3, belongs to the lipocalin family [7] and, like many other endogenous biomarker molecules, it is not produced by only one cell type. This ubiquitous 25-kDa protein is secreted by various types of human tissues, including those of the gastrointestinal tract, respiratory tract and kidneys. It is an acute phase protein, originally purified from human neutrophils, and has been found to be induced in most tissues exposed to microorganisms and in epithelial cells during inflammation [8]. NGAL is likely to play a critical role in host defense by chelating iron–siderophore complexes that enhance microbial growth [9, 10]. In kidneys, NGAL is secreted into the urine by the thick ascending limb of loop of Henle and collecting ducts of the kidney, with synthesis occurring in the distal nephron [11, 12]. Because of its small molecular size, NGAL is freely filtered and can be easily detected in urine.
Urinary NGAL (uNGAL) correlates with the degree of acute injury [13] and significantly increases in patients with progressive but not stable kidney failure [9]. NGAL has been identified as being one of the most important markers of acute kidney injury. A recent retrospective study of uNGAL in kidney transplant patients revealed that this molecule was also significantly increased in subjects with tubular pathologies [14]. The role of uNGAL as a predictive biomarker of nephrotoxicity was confirmed in patients with contrast [15] and cisplatin nephropathy [16]. However, to date, there have been almost no data published on the effect of CsA treatment on uNGAL levels, although serum NGAL (sNGAL) levels have been reported to be increased in renal allograft recipients receiving CsA treatment.
Given this background, we have attempted to determine whether the serum and urinary levels of NGAL change during CsA treatment and whether they may be seen as early indicators of CsA toxicity. To this end, the aim of this study was to determine the effects of CsA treatment on the serum and urinary levels of NGAL in steroid-dependent nephrotic children during their year of CsA treatment.
Patients and methods
We enrolled all of the nephrotic children who were diagnosed with steroid-dependent NS in our Department between January 2007 and December of 2008 and received CyA (Neoral, Novartis) therapy.
The study group (I) consisted of 19 children with steroid-dependent NS. All of these children underwent kidney biopsy, which revealed 16 cases of MCNS and three cases of focal segmental glomerulosclerosis (FSGS). The examined children (14 boys, 5 girls), aged 9.46 ± 5.52 years, were in subsequent proteinuria relapse (mean number of relapses 4.4 ± 1.4) at the time of prednisone dose reduction. At the time of the study, the children appeared to be infection-free, with a normal body temperature (<37°C) and a C-reactive protein (CRP) concentration that did not exceed 0.5 mg/dl.
In addition to receiving prednisone (0.5–1.0 mg/kg body weight on alternate days), the study group received CsA at an initial dose of 5–6 mg/kg body weight daily (max. 250 mg/24 h) in two divided doses as well as angiotensin-converting enzyme inhibitor (ACE-I) (0.05–0.1 mg/kg body weight daily). The dose of ACE-I remained constant throughout the whole study period. The serum concentration of CsA should not have exceeded 150 ng/ml during the first 6 months and 100 ng/ml during the subsequent months of treatment. In patients who had CsA-induced remission, CsA was administered at the dose that achieved the lowest possible level that maintained remission. All of the children of the study group were examined four times: at proteinuria relapse [IA; protein/creatinine (cr) ratio 9.5], prior to CsA and ACE-I treatment, and after 3 months (IB), 6 months (IC) and 12 months (ID) of CsA and ACE-I administration. The prednisone dose following proteinuria regression (2–3 weeks) was reduced to (mean) 0.6 ± 0.2 mg/kg body weight on alternate days, while the CsA dose was gradually decreased according to the serum concentration. All the patients responded to CsA during the first weeks of treatment. None of the patients was steroid- or cyclosporine A-resistant. The clinical parameters of the patients, including proteinuria, platelet count, serum albumin, creatinine (cr), cholesterol, triglyceride, CRP, and presence of hypertension, were closely followed.
The control group (II) consisted of 18 healthy children (10 boys, 8 girls) aged 3–15 years who were diagnosed on the basis of primary nocturnal enuresis and a negative history of chronic disease. These children were not receiving any medication at the time of the examinations. The urine osmolality of patients in the control group was in normal range.
All of the blood and urinary samples were collected between 7 and 8 a.m. (in children with SDNS, it was before the morning dose of CsA). Serum and urine measurements of NGAL were performed in samples frozen at −70°C. Patients with pathological leukocyturia were excluded from the study.
NGAL ELISA assay
The level of NGAL was measured in the blood and urine using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (BioPorto Diagnostics, Gentofte Denmark) according to the manufacturer's instructions. All specimens were diluted to obtain the concentration for the optimal density according to the instructions of the ELISA kit. The enzymatic reaction was quantified in an automatic microplate photometer. Both serum and urinary NGAL levels were expressed in nanograms per millilitre. The detection limit was <0.1 ng/ml.
Urinary creatinine concentration was used to normalize the NGAL measurements to account for the influence of urinary dilution on its concentration. The urinary levels of creatinine were analyzed by Jaffé’s method. The uNGAL levels were expressed as urinary NGAL/cr ratio in nanograms per millilitre creatinine. The mean intra- and inter-assay coefficients of variation (CV) for NGAL were 3.4 and 12.6%, respectively.
Trough CsA level (just before the patient takes the morning CsA dose) was estimated by means of the immunofluorescence method in polarized light, using monoclonal antibodies. The serum albumin level was measured on a Hitachi analyzer, and a biuretic method was used to assess the protein level in the urine. The estimated glomerular filtration rate (eGFR) was calculated from the Schwartz formula: eGFR = k × G (cm)/Lcr (mg/dl), where k is the age-dependent coefficient (0.55 in boys <12 years of age and girls of any age, 0.70 in boys >12 years), G is growth and Lcr is the level of creatinine in the serum.
Data analysis was performed using the computer program Statistica 8.0 (Statsoft, Tulsa, OK). Nonparametric statistics were chosen as the patient population of this study was small. Statistical analysis was performed using the non-parametric Mann–Whitney U test. Differences between the treatments were analyzed by Friedman’s analysis of variance (ANOVA) for repeated measures. Correlations between NGAL and other variables were evaluated by Pearson’s or Spearman’s test as appropriate. The study was approved by the Ethics Committee of the Medical University of Białystok in accordance with the Declaration of Helsinki.
Results
The demographic, clinical, and laboratory data on the NS patients of the healthy controls are shown in Table 1. The age and sex of the NS patients did not differ from those of the healthy controls (p > 0.05). The mean duration of NS was 4.9 ± 4.34 years. The serum albumin level and the protein/cr ratio of NS children (I) during proteinuria relapse (A), i.e. before the initiation of CsA therapy, were lower and higher, respectively, than those of the control group (p < 0.01). During CsA therapy (examinations B and C), these parameters were close to those observed in healthy children (II) (p > 0.05). During treatment, the serum CsA level decreased proportionally to dose reduction. Nephrotic patients (in all groups) had higher cholesterol levels than the controls (p < 0.01).
Serum NGAL levels in patients from group IA did not differ from those of the healthy controls (median 7.8 vs. 7.3 ng/ml, p < 0.05). After 3 and 6 months of CsA treatment, the sNGAL levels of the patients were three- and as much as sixfold higher than those of healthy controls (p < 0.01).
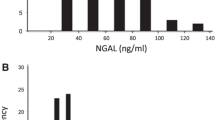
The sNGAL levels also increased significantly during CsA adjustment (Friedman ANOVA (n = 19, df3) = 35.40000, p < 0.00001; Fig. 1a). The highest level of sNGAL was found in examination IC, after 6 months of high-dose CsA therapy. After 12 months of CsA therapy, the sNGAL had decreased, and the serum concentration of CsA was also lower. We found a positive correlation between the serum levels of NGAL and CsA at examinations B and C (r = 0.52, p < 0.05 and r = 0.73, p < 0.001, respectively).
The uNGAL/cr ratio in NS children increased during CsA treatment (Fig. 1b), with the differences in the uNGAL/cr ratio between the different examinations being statistically significant [Friedman ANOVA (n = 19, df3) = 20.59239, p < 0.00013). The median uNGAL/cr ratio in control subjects and NS patients before CsA treatment was 4.2 and 5.12 ng/mg cr, respectively, and was significantly lower in the controls (p < 0.05). During the course of CsA treatment (1 year), we observed an increase in uNGAL/cr. The differences between the three examinations during CsA treatment of the NS patients and the healthy controls were statistically significant (IB vs. II, p < 0.001; IC vs. II, p < 0.001; ID vs II, p < 0.001). The urinary NGAL/cr ratio positively correlated with CsA serum concentration in examination IB, IC, and ID (respectively: r = 0.47, p < 0.05, r = 0.49, p < 0.05; r = 0.42, p < 0.05). No correlation between the uNGAL level and the CsA dose was found.
The results were similar when we checked the levels of uNGAL uncorrected for urine creatinine. The concentration of uNGAL increased during the CsA treatment: the correlation was weak in examination IA, but a statistically significant correlation with CsA level was found at all examinations.
The level of sNGAL, but not uNGAL and the uNGAL/cr ratio was found to be directly and positively correlated with serum creatinine values (r = 0.33, p < 0.001) and, inversely, with eGFR (r = −0.22, p < 0.05). We also showed that sNGAL levels were not correlated with the uNGAL/cr ratio.
When cyclosporine nephropathy (CN) was defined as an increase in serum creatinine by >50% of the baseline level, the prevalence of CN was 42% after 3 months, 52% after 6 months, and 57% after 12 months of CsA treatment. The sNGAL was significantly higher in patients with CN, starting from the third month of treatment (p < 0.05). When we defined CN as a 50% increase in creatinine level from baseline values, we calculated sensitivities, specificities, positive predictive value (PPV), negative predictive value (NPV), and the area under the curve (AUC) for sNGAL and the uNGAL/cr ratio of a respective 50% serum and uNGAL increase to predict CN. The diagnostic characteristics of sNGAL and the uNGAL/cr ratio for predicting CN are shown in Table 2.
The analysis indicated that the sNGAL assay did not appear to be predictive of CN, because the receiver operating curve (ROC) curve was quite close to the diagonal line (which indicates the "null hypothesis" of no predictive ability) (Fig. 2). The uNGAL/cr ROC curve looked better, but the AUC was still not significantly different from 0.5, so it remains unclear whether uNGAL is a good predictor of CN.
Finally, we analyzed how well the combination of both serum and urinary NGAL can predict CN, based a logistic model that used sNGAL and uNGAL as predictor variables. The results of this analysis indicate that there was no benefit to using both sNGAL and uNGAL together as predictors as the resulting ROC curve was no better than that found for uNGAL alone.
Discussion
Nephrotoxicity of CsA is observed not only in transplanted kidneys but also in patients who received CsA treatment after other organ transplants or those treated for autoimmune diseases. Acute nephrotoxicity is reversible and results from an imbalance in the release of vasoactive substances. It causes vasoconstriction of both the afferent and, to a greater degree, the efferent arterioles, leading to a decreased eGFR and an increase in renal vascular resistance. Typical changes that appear on kidney biopsy are vacuoles in the proximal and distal tubular cells and damage to the glomerular capillaries [17]. In chronic CsA nephrotoxicity, kidney biopsy shows renal tubular atrophy and arteriolopathy with fibrosis and arteriolar hyalinosis.
Although the degree of tubulointerstitial injury is observable in kidney biopsy, this process is invasive and difficult to be repeated systematically, especially in children. Therefore, it is of major clinical value to identify potential biomarkers in patients’ blood or urine that can predict the onset of tubulointerstitial damage.
The NGAL level has recently become an early indicator of kidney injury. It represents one of the most promising future biomarkers in acute kidney injury (AKI). However, recent studies also suggest a possible role in many other physiological and pathological states in clinical nephrology [18]. NGAL plays an important role in iron metabolism [10], innate immunity to bacteria [9], and kidney development [19]. There is a growing body of literature which suggests that urinary and/or serum NGAL may be a good predictor of eGFR in chronic kidney disease [20]. Plasma and urinary NGAL levels have been found to correlate well with residual renal function and serum creatinine in patients with autosomal dominant polycystic kidney disease [21]. Brunner et al. found a positive correlation between uNGAL and renal disease activity and the chronicity score based on renal histology in childhood-onset systemic lupus erythematosus [22]. These results were also confirmed by Suzuki et al. [23]. An increased uNGAL level was also postulated to be an early predictor of severity of the microangiopathic disease and secondary to associated tubular damage in diarrhea-associated hemolytic uremic syndrome [24]. Ding et al. showed that uNGAL represents an early biomarker for the degree of chronic tubulointerstitial injury in patients with immunoglobulin (Ig)A nephropathy [25]. These authors also found a strong correlation between the uNGAL/cr ratio and both clinical and histological disease activity. NGAL has also been recognized as one of the earliest and most robustly induced genes and proteins in the kidney undergoing ischemic or nephrotoxic injury [11], and it has been found to be a marker of kidney function in nephrotoxic injury following cisplatin administration [16].
Its role as a marker has also been confirmed in kidney transplant recipients with delayed graft function and acute rejection episodes [26, 27]. Malyszko et al. found a correlation between sNGAL, creatinine level, and eGFR and suggested that NGAL should be investigated as an early marker of kidney function impairment both in patients with chronic kidney disease and transplant recipients [27]. However, despite reports in the literature on the role of NGAL as a marker of tubulointerstitial damage, there are almost no data available on the effect of calcineurin inhibitors on NGAL concentration. The only investigation on this subject was performed by Malyszko et al., who found a strong positive correlation between sNGAL and calcineurin inhibitor serum concentration [27].
The results reported here confirm that both serum and urinary concentrations of NGAL change during the course of CsA treatment. In NS children experiencing a relapse of the disease, the sNGAL level was similar to that of the healthy controls, despite the presence of heavy proteinuria in the NS children. During the course of CsA treatment, the sNGAL increased significantly, and the values were higher than those in the controls and in the NS children before the treatment. The highest level of sNGAL was found after 6 months of treatment with CsA, when the CsA level in serum was >100 ng/ml. In examinations B and C, a positive correlation between sNGAL and CsA serum level was found.
The uNGAL pattern during the CsA treatment was similar between the NS children and the controls, however the uNGAL level was higher in nephrotic children before CsA treatment than in the healthy controls. Little data are available on the influence of proteinuria on uNGAL level. Kuwabara et al. found elevated uNGAL in five cases of NS at presentation, which decreased after the treatment [28]. Bolignano et al. found a positive correlation of uNGAL and serum creatinine level in proteinuric patients with idiopathic glomerulonephritis [29, 30]. Nevertheless, no data of NGAL concentration in nephrotic children are as yet available. In this study, we did not analyze the influence of proteinuria on NGAL because most of children were in complete remission.
The sources of plasma and uNGAL still require further clarification. NGAL was originally isolated from the supernatants of activated human neutrophils, but it is also expressed at low levels in the kidneys, trachea, lungs, stomach, and colon [31, 32]. As an acute phase protein, NGAL is rapidly secreted into the serum of patients with acute bacterial infections. Additional factors, such as hypertension, diabetes mellitus, peripheral inflammation disease, or urinary tract infection, which may also affect the NGAL level. In our study, we excluded patients with diabetes mellitus, urinary tract infection, and any other inflammatory diseases. However, it is possible that extrarenal tissue may make an important contribution to increased NGAL levels. The possible sources of sNGAL may be activated neutrophils/macrophages, inflammed vasculature, or endothelial dysfunction [33].
Most studies that have investigated the correlations of NGAL and parameters of kidney function have found a correlation between uNGAL and serum creatinine level, eGFR, and proteinuria. We also confirmed the positive correlation between sNGAL and creatinine and the negative correlation between sNGAL and eGFR, which is in agreement with results reported by other authors [20, 27].
We found that sNGAL and uNGAL levels increase to a greater extent in patients with a ≥50% increase in serum creatinine. In our patients, the eGFR, calculated according to the Schwartz formula, decreased from 154.2 ml/min before CsA treatment to 129.8 ml/min at the end of the 1-year treatment course. Similarly, the serum creatinine concentration increased from 0.4 to 0.65 mg/dl by the end of the treatment, which was a >50% increase.
Hinze et al. [34] confirmed that uNGAL may be predictive of worsening lupus nephritis in childhood-onset systemic lupus erythematosus (SLE) and that plasma NGAL may be predictive of worsening global and renal disease activity. Mitsnefes et al. [35] confirmed an inverse correlation between plasma NGAL levels and GFR in other types of chronic kidney disease. The reason why levels of uNAGL and sNAGL increase much more in patients with higher creatinine levels is currently a subject of speculation. It has been proposed that kidney injury results in increased NGAL mRNA expression in distant organs, especially in the liver and lungs and that the overexpressed NGAL protein may constitute a distinct systemic pool [36]. Additionally, in any kind of kidney injury, a decrease in GFR would decrease the renal clearance of NGAL, with its subsequent accumulation in the systemic circulation.
In our study, we did not find any correlation between the level of NGAL in the serum and urine in either the controls or the NS patients at all examinations. It is therefore possible that CsA influences serum and urinary NGAL via two different mechanisms.
Plasma NGAL is freely filtered by the kidney glomerulus, but it is largely reabsorbed in the PTs by efficient megalin-dependent endocytosis [12]. The results of an experimental study with labeled NGAL confirmed this process: urine became enriched with NGAL in the proximal tubule but the NGAL did not appear in the urine of the animals [37]. The urinary NGAL was originally proposed to originate in the PT epithelium [11], but subsequent studies indicated that the main sites of NGAL synthesis in the kidney are the loop of Henle and collecting ducts [32]. Urinary excretion of NGAL is likely to occur only when there is concomitant proximal renal tubular epithelium that precludes NGAL reabsorption or increases novo NGAL synthesis [11].
There is an emerging body of literature suggesting that NGAL may be a sensitive and early marker of chronic kidney disease [35]. Bolignano et al. showed that NGAL values are increased in patients with diabetic nephropathy before the appearance of pathological proteinuria [38]. These authors suggest that uNGAL may be a very early biomarker of the “tubular phase” of diabetic disease that precedes the manifestation of classic glomerular lesions. Urinary NGAL has been confirmed as an indicator of tubulointerstitial disease in a number of other investigations. Schaub et al. showed that uNGAL was significantly elevated in patients with tubulitis, even those in the subclinical phase, or with other tubular pathologies [14]. Ding et al. suggests that uNGAL may be secreted from the PT epithelial cells within damaged tubules to induce re-epithelization [25]. The hypothesis that NGAL may play the same role in repairing damaged tubules was proposed by Mishra et al. [39]. This mechanism is also possible in CN nephrotoxicity.
The results of the ROC curve indicate that the uNGAL/cr ratio is a better predictor of CN than sNGAL level. However, the AUC of the uNGAL/cr ratio was not significantly different from 0.5; consequently, we cannot be sure that uNGAL is a good predictor of CN.
It is likely that the uNGAL level reflects local kidney injury. It is also a non-invasive approach, what is really important when treating children, and it is also free of interfering proteins [40]. On the other hand, sNGAL does not need to be corrected for urine creatinine concentration. Data on the clinical value of serum and urinary NGAL are not unequivocal. Most authors suggest that uNGAL is better for predicting worsening renal function. Mishra et al. [13] showed that uNGAL had an impressive 100% sensitivity and 98% specificity in the early diagnosis of acute kidney injury. The plasma NGAL sensitivity and specificity were somewhat lower in this study. Similar results in adults after cardiac surgery were demonstrated by Wagener et al. [41]. However, in our study, both the sensitivity and specificity of uNGAL was better than sNGAL, which is in agreement with the results of Bachorzewska-Gajewska et al. [42]. In our analysis, there was no benefit to using both the serum and urine NGAL together as predictors, as the ROC curve with both molecules was no better than the one for uNGAL alone.
In conclusion, we found that both serum and urinary NGAL concentration increases in our NS pediatric patients during the course of CsA treatment. However, this is only preliminary communication, and it has a number of limitations. The major limitation is the number of patients, which is relatively small and does not allow us to draw an unequivocal conclusion. In addition, the results of the ROC curve and AUC analysis does not allow us to conclude that NGAL is a good predictor of CN. Finally, we could not compare the examined parameters with the diagnosis based on histological results.
The NGAL concentration may be influenced by many clinical situations, such as chronic hypertension, systemic infection, and inflammatory conditions. These cannot be excluded with certainty in our study, although they are not so frequent in children as in adults. All our patients were treated with ACE inhibitors, which may theoretically influence the NGAL level; however, there is as yet no data available in the literature in this area. We suggest that the influence, if any, is not significant because most of our patients were treated with ACE inhibitors before commencing on the CsA treatment, and the level of sNAGL and uNGAL/cr increased significantly during the course of CyA treatment. However, it should always be considered that the rise in uNGAL level may not only reflect renal tubular cell damage but that it may, in addition, originate from extrarenal sources.
References
Vieira JM Jr, Noronha IL, Malheiros DM, Burdmann EA (1999) Cyclosporine-induced interstitial fibrosis and arteriolar TGF-beta expression with preserved renal blood flow. Transplantation 68:1746–1753
Myers BD, Ross J, Newton L, Luetscher J, Perlroth M (1984) Cyclosporine-associated chronic nephropathy. N Engl J Med 311:699–705
Whiting PH, Thompson AW, Blair JT, Simpson JG (1982) Experimental cyclosporin A nephrotoxicity. Br J Exp Pathol 63:88–94
Burdmann EA, Andoh TF, Lindsey J, Russell J, Bennett WM, Porter G (1994) Urinary enzymes as biomarkers of renal injury in experimental nephrotoxicity of immunosuppressive drugs. Ren Fail 16:161–168
Bazzi C, Petrini C, Rizza V, Arrigo G, Napodano P, Paparella M, D'Amico G (2002) Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant 17:1890–1896
Machiguchi T, Yoshida H, Yonemoto S, Minakata T, Nomura K, Muso E, Tamura T, Sasayama S (1999) Does circulating erythropoietin reflect progression of IgA nephropathy? Comparison with urinary N-acetyl-beta-D-glucosaminidase. Nephrol Dial Transplant 14:635–640
Flower DR (1996) The lipocalin protein family: structure and function. Biochem J 318:1–14
Kjeldsen L, Cowland JB, Borregaard N (2000) Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta 1482:272–283
Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–921
Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK (2002) The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 10:1033–1043
Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P (2003) Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14:2534–2543
Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J (2007) Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18:407–413
Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365:1231–1238
Schaub S, Mayr M, Hönger G, Bestland J, Steiger J, Regeniter A, Mihatsch MJ, Wilkins JA, Rush D, Nickerson P (2007) Detection of subclinical tubular injury after renal transplantation: comparison of urine protein analysis with allograft histopathology. Transplantation 84:104–112
Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH 3rd, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P (2007) NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 22:2089–2095
Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P (2004) Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 24:307–315
Mihatsh MJ, Gudat F, Ryffel B, Thiel G (1994) Cyclosporine nephropathy. In: Tischer CC, Brenner BM (eds) Renal pathology with clinical and functional correlations. JB Lippincott, Philadelphia, pp 1641–1681
Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G, Ronco C (2009) NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. doi:10.1007/s11255-009-9608-z
Gwira JA, Wei F, Ishibe S, Ueland JM, Barasch J, Cantley LG (2005) Expression of neutrophil gelatinase-associated lipocalin regulates epithelial morphogenesis in vitro. J Biol Chem 280:7875–7882
Bolignano D, Lacquaniti A, Coppolino G, Campo S, Arena A, Buemi M (2008) Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res 31:255–258
Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M (2007) Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 27:373–378
Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, Mishra J, Devarajan P (2006) Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum 54:2577–2584
Suzuki M, Wiers KM, Klein-Gitelman MS, Haines KA, Olson J, Onel KB, O'Neil K, Passo MH, Singer NG, Tucker L, Ying J, Devarajan P, Brunner HI (2008) Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol 23:403–412
Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, Jain A, Bullington N, Devarajan P, Investigators of the HUS-SYNSORB Pk Multicenter Clinical Trial (2006) Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol 21:989–994
Ding H, He Y, Li K, Yang J, Li X, Lu R, Gao W (2007) Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol 123:227–234
Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P (2006) Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6:1639–1645
Malyszko J, Malyszko JS, Bachorzewska-Gajewska H, Poniatowski B, Dobrzycki S, Mysliwiec M (2009) Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant Proc 41:158–161
Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, Ebihara K, Omata M, Satoh N, Sugawara A, Barasch J, Nakao K (2009) Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int 75:285–294
Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M (2008) Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant 23:414–416
Bolignano D, Coppolino G, Lacquaniti A, Nicocia G, Buemi M (2008) Pathological and prognostic value of urinary neutrophil gelatinase-associated lipocalin in macroproteinuric patients with worsening renal function. Kidney Blood Press Res 31:274–279
Cowland JB, Borregaard N (1997) Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics 45:17–23
Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, Nicholas T, Devarajan P, Barasch J (2006) Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens 15:442–449
Okusa MD (2002) The inflammatory cascade in acute ischemic renal failure. Nephron 90:133–138
Hinze CH, Suzuki M, Klein-Gitelman M, Passo MH, Olson J, Singer NG, Haines KA, Brenner HI (2009) Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum 60:2772–2781
Mitsnefes MM, Kathman TS, Mishra J, Kartal J, Khoury PR, Nickolas TL (2007) Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol 22:101–108
Devarajan P (2008) Neutrophil gelatinase-associated lipocalin—an emerging troponin for kidney injury. Nephrol Dial Transplant 23:3737–3743
Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J (2005) Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115:610–621
Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, Nicocia G, Buemi M (2009) Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res 32:91–98
Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P (2004) Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15:3073–3082
Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P (2008) Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 3:665–673
Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT (2006) Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 105:485–491
Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Poniatowski B, Pawlak K, Dobrzycki S (2008) NGAL (neutrophil gelatinase-associated lipocalin) and cystatin C: are they good predictors of contrast nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine? Int J Cardiol 127:290–291
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wasilewska, A., Zoch-Zwierz, W., Taranta-Janusz, K. et al. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of cyclosporine nephrotoxicity?. Pediatr Nephrol 25, 889–897 (2010). https://doi.org/10.1007/s00467-009-1397-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-009-1397-1