Abstract
We hypothesized that neutrophil gelatinase-associated lipocalin (NGAL) is an early predictive biomarker of disease activity in lupus nephritis. NGAL in serial plasma (PNGAL) and urine (UNGAL) samples was measured by enzyme-linked immunosorbent assay (ELISA) in 85 participants with pediatric systemic lupus erythematosus (pSLE), healthy children (n = 50), and children with juvenile idiopathic arthritis (JIA) (n = 30). Disease activity was measured by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). Plasma and urinary NGAL were significantly increased in subjects with pSLE compared with those with JIA or with healthy controls (all p < 0.03), and unrelated to subjects’ age, weight, or height. Plasma and urinary NGAL were stable in pSLE subjects with unchanged disease activity. The pSLE subjects with worsening global or renal disease activity had a mean ± standard error (SE) increase of UNGAL (in ng/ml) of 11.5 ± 6.4 or 36.6 ± 12.1 (p < 0.01), corresponding to a 156% or 380% increase, respectively. PNGAL increased with worsening disease but to a much lesser degree than UNGAL [global disease activity (mean ± SE): 7.3 ± 6.2 or 21%; renal disease activity: 20.2 ± 6.0 or 51%; both p = not significant]. In conclusion, NGAL in urine but not in plasma represents a novel biomarker for renal disease activity in pSLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal involvement is one of the main determinants of poor prognosis of systemic lupus erythematosus (SLE) [1] and is more frequently encountered in children than in adults with SLE. Currently available renal biomarkers, i.e. measures of the degree of SLE renal disease activity and severity, are too insensitive to allow for early identification of patients with active SLE nephritis, prohibiting timely initiation of therapy to avoid permanent renal damage [2]. Randomized clinical trials in SLE are hindered by the lack of high-quality biomarkers to verify the effects of therapies within a short period of time [3].
Neutrophil gelatinase-associated lipocalin (NGAL) is a member of the lipocalin family of proteins that has been extensively studied in acute kidney injury [4]. NGAL is one of the most robustly expressed proteins in the kidney following ischemic or nephrotoxic injury in both animals [5–9] and humans [10–13]. Importantly, a recent prospective pediatric study demonstrated that concentrations of NGAL in urine and plasma represent novel, sensitive, and specific biomarkers for early identification of acute kidney injury following cardiac surgery [13]. Our previous preliminary data also suggest that urinary NGAL levels are markers of renal disease activity and renal damage in children with pediatric SLE (pSLE) [14].
Plasma NGAL concentrations are elevated in patients with atherosclerosis, ovarian cancer, and systemic vasculitis, including Kawasaki syndrome [15–18], whereas plasma NGAL levels in pSLE have not been investigated. In this study, we hypothesized that both urinary and plasma NGAL change with renal disease activity. The purpose of this study, therefore, was to assess the relationship of urine and plasma NGAL levels with disease activity in pSLE with a special emphasis on nephritis.
Methods
Patients
With approval of the institutional review boards of the participating institutions, children fulfilling American College of Rheumatology Classification Criteria for SLE [1] prior to the age of 16 years were studied during routine visits to the pediatric rheumatology and lupus clinics. A convenience sample of 30 children diagnosed with juvenile idiopathic arthritis (JIA) [19] were recruited as disease controls. Samples of healthy controls (n = 50) were obtained from the Cincinnati Genomic Control Cohort assembled by the Cincinnati Children’s Hospital Medical Center.
Study design
The medical record was reviewed to screen for preexisting renal disease in subjects with JIA and to obtain pSLE-specific information. Review of system information and the results of routine laboratory testing at the time of the study visits were recorded. Relevant demographic data of all participants were obtained, as was information on medication regimens. NGAL levels were assessed cross-sectionally in all subjects (pSLE subjects, JIA controls, healthy controls) and over time in the 52 pSLE subjects with available NGAL testing over time in 3 month intervals.
Laboratory testing for NGAL
NGAL levels in urine and plasma were quantified by enzyme-linked immunosorbent assay (ELISA) using an NGAL ELISA kit (Kit 036; AntibodyShop, Grusbakken, Denmark) that specifically detects human NGAL. The assay was performed as per the manufacturer’s protocol. Briefly, 100 μl of NGAL standards or diluted samples (urine or plasma) were applied to the precoated microwells in duplicates. Microwells were then incubated for 1 h at room temperature and then washed with washing buffer. In succession, biotinylated NGAL antibody and horseradish peroxidase (HRP)-streptavidin were incubated in the wells for 1 h each with shaking at 200 rpm. Tetramethylbenzidine dihydrochloride (TMB) substrate was added for 10 min in the dark before adding stop solution. Finally, NGAL concentration was measured at 450 nm wavelength in each well, with reference reading at 620 nm in blank wells. The intra-assay coefficients of variation were 2.1% (range: 1.3–4.0%) and 3.0% (range: 1.2–4.0%) in urine and plasma, respectively. Interassay variation was 9.1% (range: 6.8–18.1%) and 8.2% (range: 2.2–11.2%) in urine and plasma, respectively. Urine creatinine was measured using quantitative colorimetric Microplate Assay Kit (Oxford Biomedical Research, Oxford, MI, USA) to standardize urinary NGAL for changes in urine concentration. Urinary NGAL (UNGAL) excretion is presented as the amount of urinary NGAL in nanograms per milliliter (ng/ml) urine (UNGAL-ml), as well as urinary NGAL in nanograms per milligram (ng/mg) of urine creatinine to correct for differences in NGAL due to urine dilution (UNGAL-crea). The concentration of NGAL in the plasma (in ng/ml plasma) is referred to as PNGAL. All measurements were made in triplicate and in a blinded fashion.
Information obtained for pSLE subjects
Changes in global disease activity were measured by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI-2K) [20]. Extrarenal disease activity was defined as the summary score of the SLEDAI-2K excluding the scores accrued in the renal domain of the SLEDAI-2K. Renal disease activity was defined as the sum of the SLEDAI-2K scores accrued in the renal domain of the measurement. Higher SLEDAI-2K scores represent more active disease.
All participating centers perform kidney biopsies on any pSLE patient who has abnormal urinalyses that cannot be explained by mechanisms other than SLE. Because some renal biopsies were obtained prior to the introduction of the new classification system of SLE nephritis [21], the original system was used [22].
Laboratory testing recorded for the study included blood urea nitrogen (BUN), serum creatinine, urinalysis and urine microscopy, urinary protein to creatinine ratio, serum complement levels C3 and C4 (categorized as normal or low), hemoglobin, erythrocyte sedimentation rate (ESR), and titers of anti-dsDNA antibodies (Crithidia or Farr assay; categorized as negative or positive/elevated).
Worsening (flare) of overall (renal) pSLE disease course between visits was measured in two ways. First, we recorded physician-rated worsening of global (renal) disease as defined by an increase in the scores of the disease activity estimate on a physician visual analog scale (MD-VAS; range 0–10). Second, we determined any increase in the scores of the overall (renal domain) score of the SLEDAI-2K. Global disease activity was considered unchanged or improved in cases where the MD-VAS or the SLEDAI-2K suggested that the pSLE subject’s disease was stable or improved, respectively. Additionally, physicians completed a Likert scale to indicate whether there were global (renal) flares, stable global (renal) disease, or improving global (renal) disease between study visits.
Control population
Plasma and urine specimens from healthy controls were generously donated by the Cincinnati Genomic Control Cohort. Information on the review of systems as well as demographic information was available. For participants with JIA, the results of routine urinalyses and serum creatinine testing ordered to screen for nonsteroidal anti-inflammatory drug (NSAID)- and/or methotrexate-related toxicity were recorded. In addition, information pertaining to subject demographics and the JIA core response variables was obtained [23], including ESR, physician-rated disease activity (VAS 0–10), and the number of joints with active arthritis and those with limited range of motion.
Data analysis
EXCEL XP (Microsoft Inc., Redmond, WA, USA) and SAS 9.1 (SAS Institute Inc., Cary, NC, USA) were used for analysis. Means and standard errors (SE) values were calculated as measures of central tendency. Groups of patients were assessed for statistically significant differences using analysis of variance (ANOVA). For pSLE subjects, plasma and urinary NGAL levels, the values of laboratory parameters (serum creatinine, glomerular filtration rate, proteinuria, urinary protein to creatinine ratio, titers of anti-dsDNA antibodies, hemoglobin), and scores of disease measures [SLEDAI-2K, British Isles Lupus Activity Group (BILAG) index, Systemic Lupus International (SDI)] were correlated using Spearman’s correlation coefficients (r). Mixed models correcting for differences in gender and race were used to assess changes of NGAL for important differences over time in the subset of cSLE subjects with available longitudinal data. Post hoc testing was performed with the Tukey procedure.
Results
Pediatric SLE subjects
Data of 85 subjects with pSLE were available, and 52 of them had at least one follow-up visit (total number of visits: 132). The demographic information of pSLE subjects is summarized in Table 1 and results of their laboratory testing are shown in Table 2. The mean time ± SE between the first study visit and the time of renal biopsy was 2 ± 0.35 years for those subjects who had biopsies (n = 48).
At baseline, the mean ± SE of PNGAL, UNGAL-ml, and UNGAL-crea of pSLE subjects was 63.6 ± 4.7, 44.6 ± 7.3, and 29.2 ± 4.6, respectively. UNGAL was unrelated to PNGAL (r < 0.13; p = NS), whereas UNGAL-ml was strongly correlated with UNGAL-crea (r = 0.8; p < 0.0001).
JIA controls
Thirty children with JIA (female:male = 27:3; mean ± SE age 15.6 ± 0.1 years) participated in the study. There were four African American and 26 Caucasian subjects with JIA. These subjects were treated with NSAIDs alone (n = 2), methotrexate (MTX) alone (n = 8), or the combination of NSAIDs and MTX (n = 12). Eleven JIA subjects were treated with biologic medications (etanercept, abatacept, infliximab, adalimumab) alone or in combination with NSAIDs and/or MTX. The mean ± SE number of active joints and joints with limited range of motion was 2.3 ± 0.3 and 1.7 ± 0.1, respectively; ESR or C-reactive protein (CRP) levels were elevated in seven (25%) of the 24 JIA controls with available data. None of the JIA controls had a history of chronic or recent acute urinary tract infection, and all had normal urinalyses and normal serum creatinine levels.
The mean ± SE of PNGAL, UNGAL-ml, and UNGAL-crea were 60.7 ± 9.7, 24 ± 3.8, and 17.5 ± 3.1, respectively. There was no apparent relationship between UNGAL and PNGAL in JIA (r < 0.1; p = NS). PNGAL and UNGAL (UNGAL-ml or UNGAL-crea) were unrelated to physician-rated JIA disease activity (MD-VAS with range 0–10) and the weekly dose of MTX. Furthermore, NGAL in plasma or urine did not differ with exposure to NSAIDs or biologic medications.
Healthy controls
The 50 healthy children (female:male = 28:22) had a mean ± SE age of 14.8 ± 0.05 years. There were 16 African American and 34 Caucasian healthy controls. The mean ± SE of PNGAL, UNGAL-ml, and UNGAL-crea were 71.4 ± 0.6, 15 ± 0.4, and 7.9 ± 0.2, respectively.
Cross-sectional differences in NGAL levels between pSLE subjects and controls
Using ANOVA and Tukey post hoc testing, differences in NGAL levels between groups of subjects (pSLE, JIA subjects, and healthy children) were assessed. UNGAL did not differ significantly between JIA and healthy controls. Conversely, pSLE subjects had significantly higher UNGAL-ml (p < 0.003) and UNGAL-crea than did controls (p < 0.0001). With respect to PNGAL, JIA controls had significantly higher levels than did healthy controls (p < 0.03), and pSLE subjects had significantly higher levels than did subjects with JIA (p < 0.03). Irrespective of diagnosis (JIA, pSLE, or healthy), NGAL (urine or plasma) did not differ with patient weight, height, or age. Among pSLE subjects, Caucasians had a trend toward lower NGAL (urine and plasma); the same was true for male compared with female pSLE subjects (p=NS). UNGAL but not PNGAL correlated with the blood pressure of JIA and pSLE subjects (all r > 0.32; p < 0.002).
NGAL: relationship to pSLE disease features
Correlation of NGAL with select pSLE laboratory parameters and treatments
NGAL (urine and plasma) was unrelated to the daily dose of prednisone, creatinine clearance, complement C3 and C4 levels, and ESR (all r < 0.2). There was a significant correlation between the urine protein:creatinine ratio and PNGAL (Spearman correlation coefficient r 2 = 0.2; p = 0.03), UNGAL-ml (r 2 = 0.3, p = 0.003), and UNGAL-crea (r 2 = 0.43, p = 0.001). NGAL (urine and plasma) levels did not significantly differ with the use of immunosuppressive medications. There was a trend toward higher UNGAL in subjects treated with angiotensin-blocking drugs (for UNGAL-crea: 42 ± 8.2 vs. 24 ± 5.4; p = NS). Among the laboratory indicators tested, PNGAL was only weakly correlated to the pSLE subjects’ ESR (r = 0.22; p = 0.06).
Plasma NGAL and disease activity
The pSLE subjects with inactive disease (SLEDAI-2K = 0) had somewhat lower PNGAL than those with active disease (54.5 ± 10.3 vs. 65.3 ± 5.1; p = NS). PNGAL did not differ significantly between pSLE subjects with vs. without active renal disease activity (62.5 ± 7.3 vs. 64.1 ± 5.9; p = NS). There was a trend towards higher PNGAL with active vs. inactive extrarenal disease activity (65.9 ± 5.3 vs. 56.1 ± 9.5; p = NS).
Urinary NGAL and disease activity
UNGAL correlated moderately with renal disease activity as measured by the SLEDAI-2K (for UNGAL-crea: r = 0.4; p < 0.008). The mean ± SE of UNGAL-crea was 45.4 ± 11.6 with active renal disease activity and only 21.7 ± 3.7 in subjects with inactive lupus nephritis (p = 0.02); the mean ± SE of UNGAL-crea differed with active vs. inactive global disease activity (SLEDAI >0 vs. SLEDAI = 0; 31.7 ± 5.2 vs. 15.6 ± 7.3; p = 0.06). UNGAL-crea did not change with extrarenal disease activity (active vs. inactive extrarenal SLEDAI >0 vs. = 0: 65.3 ± 5.1 vs. 54.5 ± 7.3; p = 0.2).
NGAL and findings on renal biopsy
Renal damage as measured by the SDI was present in only four subjects, hence rare in this cohort. UNGAL-crea but not UNGAL-ml or PNGAL differed between groups of pSLE subjects with various degrees of renal involvement [based on World Health Organization (WHO) class] when compared by ANOVA (p < 0.02). When analyzing only the 12 subjects whose kidney biopsy was performed within 2 months of NGAL measurement, the NGAL values differed significantly between subjects with WHO class IV vs. class V lupus nephritis. The mean (SE) of PNGAL, UNGAL-ml, and UNGAL-crea with WHO class IV lupus nephritis (n = 7) was 95 (19), 60 (27), and 58 (17) respectively, compared with subjects with class V lupus nephritis (n = 5) with corresponding values of 49 (11), 16 (4), and 10 (3), respectively. There was a statistically significant difference in UNGAL-crea between class IV and class V by nonparametric ANOVA (p = 0.02).
Relation of NGAL with worsening in disease activity over time in pSLE
Changes of PNGAL in relation to worsening disease activity are summarized in Fig. 1 and those of UNGAL are shown in Fig. 2 using UNGAL-ml as an example (as UNGAL-crea showed similar relationships to changes in disease activity).
Mean ± standard error (SE) of absolute changes in plasma neutrophil gelatinase-associated lipocalin (NGAL) are depicted in the upper panel and the mean ± SE of percentage changes of plasma NGAL are shown on the lower panel with changes in global and renal disease activity. Plasma NGAL increased with worsening of global but less pronounced as with worsening of renal disease activity. Increases in plasma NGAL varied widely, and after correction for differences in NGAL levels for race and gender in mixed model analysis, none of the changes reached statistical significance at p < 0.05 in mixed-model analysis. Also see legend, Table 2
Mean ± standard error (SE) of absolute levels of neutrophil gelatinase-associated lipocalin (NGAL) (ng/ml urine) in the urine increased significantly with worsening of renal disease activity (p < 0.01), irrespective of the external standard [physician visual analog scale (MD-VAS) assessment or renal domain score of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI-2k)] chosen (upper panel). The same was true when the mean ± SE of relative changes (%) were considered (lower panel). Although urinary NGAL increased with worsening of global or overall disease activity, such changes were less pronounced and only reached statistical significance when the MD global assessment was used as external standard. Also see Table 2 legend; * p < 0.05 based on mixed models correcting for race, gender; ** p < 0.01 based on mixed models correcting for race, gender
PNGAL and worsening of pSLE
Similar trends were observed for both relative (% change of PNGAL) and absolute changes of PNGAL over time. PNGAL increased particularly with worsening renal disease activity (MD-rated, SLEDAI-renal). For example, PNGAL increased by 40% or a mean ± SE (ng/ml) 51 ± 19.4 with worsening SLEDAI-renal scores. Irrespective of the external standard considered (MD-VAS, SLEDAI-2K), none of these PNGAL changes reached statistical significance (Fig. 1).
UNGAL and worsening of pSLE
UNGAL levels often increased significantly with worsening of global disease activity but particularly increased with worsening renal disease activity (Fig. 2), irrespective of the external standard used (MD-VAS, SLEDAI-2K). For example, with increasing renal disease activity (renal SLEDAI-2K) mean ± SE of UNGAL-ml and UNGAL-crea rose by 380% and 125% or 27 ± 12 and 9 ± 8, respectively (all p < 0.01).
NGAL and physician-rated clinically significant changes in renal disease
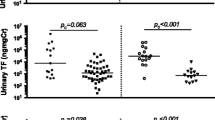
A Likert scale was completed by the treating physician to indicate whether pSLE subjects’ renal disease had improved (n = 16), worsened (n = 8), or was stable (n = 17) between visits. The mean ± SE of PNGAL decreased by 10.6 ± 2.0 with renal improvement, decreased by 22.6 ± 11 with renal flare, and increased by 4.9 ± 0.7 when renal disease was considered to be stable. The respective changes of UNGAL-ml were −56.8 ± 6.0, +26.8 ± 12.3, and −14.9 ± 0.5; and of UNGAL-crea −51.5 ± 6.2, +7 ± 7.7, and −5.6 ± 0.5. Figure 3 provides a comparison of the relative (%) changes of NGAL in plasma and urine with changes in renal disease activity as PNGAL remained relatively stable despite changes in renal disease. This is different from changes in UNGAL-ml and UNGAL-crea, which increased by 340% and 129% with renal flares, whereas with improving renal disease, UNGAL-ml and UNGAL-crea decreases of 47% and 10% were observed, respectively. On average, only small increases of UNGAL occurred in pSLE subjects whose renal disease was considered unchanged. Differences in UNGAL-ml and UNGAL-crea, but not those of PNGAL, reached statistical significance at p < 0.01 and p < 0.05, respectively.
Mean ± standard error (SE) of relative (%) changes in neutrophil gelatinase-associated lipocalin (NGAL) with changes of lupus nephritis as judged by the treating physician are depicted for (1) plasma NGAL, (2) urinary NGAL, and (3) urinary NGAL corrected for urinary creatinine. Mean ± SE of changes of NGAL in plasma were small compared with those of NGAL excreted in urine and did not reach statistical significance. * p < 0.05 based on mixed models correcting for race, gender; ** p < 0.01 based on mixed models correcting for race, gender
Changes in other renal disease measures over time
Complement levels (C3, C4) and the protein:creatinine ratio changed with disease course but to a lesser degree, as shown in Table 3.
Discussion
Our cross-sectional and longitudinal data indicate that urinary NGAL rather than plasma NGAL is closely related to worsening global or renal disease activity in pSLE. Irrespective of whether urinary NGAL excretion was standardized for urinary creatinine excretion (UNGAL-crea) or not (UNGAL-ml), worsening renal disease activity resulted in marked increases of UNGAL. Plasma concentrations of NGAL fluctuated widely in pSLE, and there was no significant increase with renal disease activity change.
Our previous research supports that UNGAL may be a new sensitive biomarker of lupus nephritis, with increased levels present in both patients with active lupus nephritis or renal damage due to pSLE. In the current cohort with its relative short disease duration of 5.8 years of subjects with renal biopsy-proven lupus nephritis, disease damage was rare, prohibiting a statistical analysis of the relationship of disease damage and NGAL. As in our previous study, UNGAL was related to disease damage in pSLE [14]. This is supported by a recent publication by Pitashny et al. who correlated UNGAL in adult SLE patients with renal parameters of disease activity and severity [24].
UNGAL elevations have been noted with several other renal diseases and are not specific for pSLE [25–27]. We speculate that UNGAL in pSLE nephritis is produced principally by the injured tubule cells, in direct proportion to the degree and severity of disease. Our studies do not rule out contributions from other cell types, such as neutrophils [15] or inflamed vasculature [28], as sources of UNGAL in pSLE. However, the fact that UNGAL excretion levels correlated with the markers of renal disease activity as well as changes in renal disease activity and involvement much stronger than with global disease activity as well as the changes in global disease activity, suggests that the renal epithelial cells are the major source of NGAL detected in urine. In addition, NGAL produced elsewhere in the body is thought to be almost completely reabsorbed by the kidneys at the level of the proximal tubule, unless there is concomitant renal injury [13, 29]. This is supported by the observation that PNGAL and UNGAL levels correlated only weakly with each other in pSLE. Additional support is provided by the observation that healthy controls and children diagnosed with JIA who, despite requiring anti-inflammatory and potentially nephrotoxic medications, have very low UNGAL levels.
Our data support previous reports that NGAL levels do not differ with patient age, gender, or race [13, 24, 29]. The trend toward higher NGAL levels among African Americans may be due to their higher prevalence of nephritis compared with the participating Caucasian patients with pSLE (28/37 = 75% vs. 22/42 = 52%; p = NS).
Previous studies have indicated that UNGAL is a predictive biomarker for acute kidney injury. UNGAL measured at 2 h after an ischemic insult are 98% sensitive and 100% specific in predicting acute kidney injury up to several days later [13]. Although our results provide firm support of NGAL changes with worsening of lupus nephritis, and our previous studies support that UNGAL is very sensitive and specific for identifying pSLE patients with biopsy-proven nephritis, active renal disease and renal damage [14], the paucity of longitudinal data did not allow us to effectively test for the predictive properties of NGAL in pSLE.
In exploratory analysis, PNGAL levels were markedly and significantly increased with neurological and vascular disease activity as measured by the BILAG index [30]. However, this was based on few observations and not the primary focus of our research. Of note, renal-specific changes of NGAL persisted irrespective of whether the SLEDAI-2K or the BILAG index was used to measure pSLE disease activity.
NGAL excretion in relationship to cyclophosphamide therapy requires further investigation, as alkylating agents are known to cause uroepithelial injury. However, corrected for renal disease activity, NGAL levels of the five subjects treated with cyclophosphamide at the time of the NGAL measurement did not differ from those of pSLE subjects who previously received cyclophosphamide or had never been exposed to the drug (data not shown).
A limitation of this study may be that renal biopsies were often not obtained in close timely relationship to the study, limiting their suitability to serve as an external standard for NGAL validation. This is because renal histology can change rapidly with therapy, and current laboratory markers are not suitable to accurately estimate the degree of lupus nephritis. However, in the limited number of subjects examined with kidney biopsies obtained within 2 months of NGAL measurement, the UNGAL levels appeared to discriminate between WHO class IV and class V lupus nephritis, UNGAL levels being significantly greater in subjects with class IV disease. However, this finding needs to be confirmed in larger studies.
Another limitation is that additional validation studies based on larger longitudinal data sets are required to construct receiver operating characteristic curves in an effort to specify clinically relevant changes of UNGAL that are indicative for SLE renal flares. Especially important will be to examine the predictive properties of NGAL, e.g. to delineate whether rises of UNGAL are indicative of future renal flares, and whether urinary NGAL levels rise before C3 levels fall or before serum creatinine or proteinuria increase.
In summary, we present evidence that UNGAL represents a high-quality biomarker for SLE renal disease. Although these data have been obtained in young patients with pSLE, NGAL measurement may also be useful for older adults with SLE given the similarities in the underlying disease processes of both pSLE and adult-onset SLE. Additional research is required to further characterize the measurement properties of UNGAL in children and adults with SLE.
References
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Ho A, Barr SG, Magder LS, Petri M (2001) A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum 44:2350–2357
Schiffenbauer J, Hahn B, Weisman MH, Simon LS (2004) Biomarkers, surrogate markers, and design of clinical trials of new therapies for systemic lupus erythematosus. Arthritis Rheum 50:2415–2422
Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J (2007) Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18:407–413
Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P (2003) Differential gene expression following early renal ischemia/reperfusion. Kidney Int 63:1714–1724
Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P (2003) Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14:2534–2543
Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P (2004) Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 24:307–315
Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P (2004) Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15:3073–3082
Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S (2003) Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab 80:365–376
Devarajan P (2005) Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr 17:193–199
Nguyen MT, Ross GF, Dent CL, Devarajan P (2005) Early prediction of acute renal injury using urinary proteomics. Am J Nephrol 25:318–326
Mishra J, Ma Q, Kelly K, Mitsnefes M, Barasch J, Devarajan P (2006) Kidney NGAL is a novel earl marker of acute injury following transplantation. Pediatr Nephrol 21:856–863
Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365:1231–1238
Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, Mishra J, Devarajan P (2006) Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum 54:2577–2584
Biezeveld MH, van Mierlo G, Lutter R, Kuipers IM, Dekker T, Hack CE, Newburger JW, Kuijpers TW (2005) Sustained activation of neutrophils in the course of Kawasaki disease: an association with matrix metalloproteinases. Clin Exp Immunol 141:183–188
Lim R, Ahmed N, Borregaard N, Riley C, Wafai R, Thompson EW, Quinn MA, Rice GE (2007) Neutrophil gelatinase-associated lipocalin (NGAL) an early–screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer 120:2426–2434
Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S (2007) Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant 22:295–296
Ohlsson S, Wieslander J, Segelmark M (2003) Increased circulating levels of proteinase 3 in patients with anti-neutrophilic cytoplasmic autoantibodies-associated systemic vasculitis in remission. Clin Exp Immunol 131:528–535
Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, Maldonado-Cocco J, Suarez-Almazor M, Orozco-Alcala J, Prieur AM (1998) Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol 25:1991–1994
Gladman DD, Ibanez D, Urowitz MB (2002) Systemic lupus erythematosus disease activity index 2000. J Rheumatol 29:288–291
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65:521–530
Churg J, Bernstein J, Glassock RJ (1995) Renal disease: classification and atlas of glomerular diseases, 2nd edn. Igaku-Shoin, New York, p 541, p xvi
Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A (1997) Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 40:1202–1209
Pitashny M, Schwartz N, Qing X, Hojaili B, Aranow C, Mackay M, Putterman C (2007) Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum 56:1894–1903
Devarajan P (2007) Emerging biomarkers of acute kidney injury. Contrib Nephrol 156:203–212
Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT (2006) Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 105:485–491
Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P (2006) Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6:1639–1645
Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK (2006) Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol 26:136–142
Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J (2005) Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115:610–621
Hay EM, Bacon PA, Gordon C, Isenberg DA, Maddison P, Snaith ML, Symmons DP, Viner N, Zoma A (1993) The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med 86:447–458
Acknowledgements
This study is supported by clinical research grant NIAMS P60 AR47784. Dr. Brunner is supported by a grant from the Alliance for Lupus Research and the CCHMC Translational Research Initiative. Dr. Devarajan is supported by grants from the NIH/NIDDK (RO1 DK53289, P50 DK52612, R21 DK060163). Dr. Wiers is supported by T32 AR007594 and the NIH Loan Repayment Program. We are indebted to our clinical coordinators Jamie Meyer-Eaton and Shannen Nelson for their assistance, Lukasz Itert for the data management, the NIAMS Tissue Repository (director: Susan Thompson; AR047363) for the sample management, Qing Ma for her technological support, and to our patients and families for their participation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, M., Wiers, K.M., Klein-Gitelman, M.S. et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol 23, 403–412 (2008). https://doi.org/10.1007/s00467-007-0685-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-007-0685-x