Abstract
Plasma phosphate levels are important in the evolution of hyperparathyroidism and ectopic calcification in chronic kidney disease (CKD). Although dietary management may be adequate to control plasma phosphate in its early stages, most patients develop hyperphosphataemia by CKD stages 3−4 and require the addition of a phosphate binder. Calcium-containing phosphate binders are the most used and cheapest binders but have fallen out of favour because of the potential for positive calcium balance and calcium toxicity. This problem may be attenuated by newer phosphate binders such as sevelamer hydrochloride and lanthanum carbonate. In this review, the role of phosphate as a uraemic toxin and the advantages and disadvantages of the currently available phosphate binders are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphate has probably the best described spectrum of toxicity of all molecules that circulate in excess in chronic kidney disease (CKD). Decreased renal phosphate excretion plays a major role in the onset of hyperparathyroidism. Furthermore, plasma phosphate levels are positively and independently correlated with an increasing risk of death from cardiovascular disease [1]. However, despite these clear associations, control of our patients’ plasma phosphate is one of our most challenging management issues, and indeed, some physicians believe that a high plasma phosphate is an inevitable consequence of CKD, accepting that good phosphate control is an impossible task.
The role of phosphate in the evolution of hyperparathyroidism
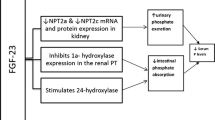
Phosphate is filtered at the glomerulus and reabsorbed in the proximal tubules, with approximately 85% of the filtered phosphate reabsorbed via the sodium−phosphate cotransporter IIa located in the proximal tubular brush-border membranes. It would be expected, therefore, that CKD would result in hyperphosphataemia. However, we now know that compensatory mechanisms act to preserve a normal plasma phosphate in early CKD. The molecules responsible for this are fibroblast growth factor-23 (FGF-23), a hormone produced by the osteocyte; and Klotho, a single-pass transmembrane protein. FGF-23 requires Klotho as an obligatory coreceptor to activate FGF signalling. Together they result in negative phosphate balance by decreasing renal tubular phosphate reabsorption and by suppressing the renal 1-α hydroxylase receptor, thereby reducing the synthesis of 1,25-dihydroxyvitamin D [1,25(OH)2D]. This, in turn, decreases gastrointestinal phosphate absorption [2].
The sequence of events that stimulate this process is not yet known: it could be that decreased Klotho expression from renal damage stimulates an increase in FGF-23 to compensate for the increasing phosphate burden, or that osteocytes sense decreased hydroxyapatite deposition and produce FGF-23 to excrete excess phosphate in the exchangeable pool. Either way, FGF-23 and Klotho act together to decrease plasma phosphate levels in early CKD so that normal phosphate levels are maintained. However, as CKD progresses, there is increasing FGF-23 resistance and phosphate retention occurs, stimulating parathyroid hormone (PTH) secretion. FGF-23 is very important because there is evidence that it is an independent predictor of vascular calcification: FGF-23 levels are raised even in the early stages of CKD, are persistently raised in dialysis patients and have been linked with increased mortality rate [3]. Phosphate-stimulated increased secretion of PTH and FGF-23 causes a vicious circle that contributes to ongoing high phosphate levels and hyperparathyroidism in two ways: firstly, PTH increases 1,25(OH)2D levels, which increase intestinal phosphate absorption; and secondly, FGF-23 decreases 1,25(OH)2D levels, thus increasing PTH secretion via the vitamin D receptors in the parathyroid glands (Fig. 1).
The role of phosphate in vascular disease
Studies in adult patients have conclusively identified that plasma phosphate is an independent predictor of mortality in CKD. This link was first demonstrated in 1998 in adult haemodialysis (HD) patients: as serum phosphate levels increased above 5.6 mg/dl (= 1.8 mmol/L), the hazards ratio for mortality increased by 6% for every 1 mg/dl (= 0.3 mmol/L) increase in serum phosphate [1]. Hyperphosphataemia has also been shown to be an independent risk factor for death in the predialysis population [4, 5]. Phosphate exerts its effect on the cardiovascular system principally by its role in vascular calcification, which leads to diastolic dysfunction, hypertension and increased cardiac work [6]. More recently, it has been established that phosphate levels in patients not already established on dialysis are also associated with vascular and valvular calcification [7].
Vascular calcification is a complex, cell-mediated process and not simply the result of passive calcium deposition. Calcium accumulation begins in vascular smooth muscle cells (VSMCs) predialysis and is proportional to the blood calcium × phosphate product [8]. In the early stages, mineralisation inhibitors such as matrix Gla-protein and fetuin limit the calcification potential. With the commencement of dialysis, VSMCs adapt to the increasing intracellular calcium load by developing vesicles that act to extrude calcium from the cell. However, in the dialysis milieu, loss of inhibitors, worsening calcium/phosphate balance and possibly other factors such as oxidative stress, overwhelm VSMC defence mechanisms, leading to VSMC apoptosis [9]. In fact, many studies have shown that approximately 25–30% of patients with CKD do not develop calcification despite exposure to the same uraemic milieu, suggesting that the balance of procalcific factors and circulating inhibitors is key in the development and progression of calcification [10].
In vitro studies using VSMC explant cultures have shown the direct causal role of phosphate in inducing and promoting vascular calcification [11, 12], and this has been confirmed in intact human vessels [8, 12]. In the presence of increased intracellular phosphate, the sodium-dependent phosphate cotransporter Pit-1 signals through Cbfa-1, an osteoblastic transcription factor, to induce osteoblastic differentiation of vascular cells [13, 14]. Exposure of VSMCs to media containing elevated levels of calcium and/or phosphate rapidly induces calcification with synergistic effects if both ions are elevated [8, 11, 12]. Apoptosis of VSMCs contributes to the accelerated calcification. Thus, phosphate can act as a signalling molecule and induce phenotypic changes in the VSMCs as well as directly contribute to the mineralisation process.
Studies of cardiovascular disease and its associations with disordered mineral metabolism in CKD in children have used surrogate markers of vascular structure, function and calcification that have been validated as reflecting mortality risk in adult studies. Carotid intima media thickness (cIMT) represents structural changes in the vessel wall, pulse wave velocity (PWV) functional changes and vascular stiffness, and electron-beam computed tomography (EBCT) and multislice CT (MSCT) scanning demonstrate direct evidence of calcification in the coronary arteries (CAC) [15–19].
Plasma phosphate has been shown to adversely affect cIMT in three paediatric studies [15–17], left-ventricular mass (LVM) in one [16] and CAC in two [15, 18]. Every 1 mmol/L increase in serum phosphate level was associated with a 0.15-mm increase in cIMT in 85 children on dialysis [15]. Calcium × phosphate product was also positively correlated with cIMT [16, 18, 19], LVM [16] and CAC [18]. Importantly, when intact vessels from children with CKD were examined, calcium-phosphate (hydroxyapatite) crystal deposition was found even in predialysis CKD, and the vessel calcium load correlated closely with the time-averaged serum Ca-PO4 product [8].
What phosphate level should we aim for?
There is an association between phosphate levels and coronary artery calcification in young adults without kidney disease [20], so it is not surprising that in patients with CKD as well, phosphate levels within the normal range are associated with a greater prevalence of vascular and valvular calcification [7]. There are, however, no clinical trials addressing the issue of plasma phosphate levels and mortality rate, although one interesting study has demonstrated that the use of any type of phosphate binder, even with phosphate levels in the normal range and therefore below levels currently recommended for phosphate binder use, is associated with decreased mortality rate in patients on hemodialysis [21]. The authors speculate that this may be due to decreased FGF-23 stimulation.
So what phosphate level should we aim for in children with CKD? It has to be remembered that plasma phosphate varies throughout childhood, falling steeply from birth until the age of 1−2 years and then continuing to fall more slowly until the age of 7 years (Fig. 2). The optimum target is not clear, but a plasma phosphate level that is persistently at the upper limit of normal implies that there are times when it is above normal. It makes sense, therefore, to try to keep the plasma phosphate around the 50th centile.
Phosphate centiles according to age. With permission from [49]
Control of plasma phosphate is very difficult to achieve, as dietary manipulation is difficult, and phosphate binders are the most unpopular of medications, associated with huge compliance issues because of the number of tablets required, frequency of administration, monotonous need to be chewed and taken with meals and size and palatability. Dialysis is rarely adequate to clear phosphate without the use of phosphate binders.
Dietary control of phosphate
A normal diet contains around 800–1,500 mg of phosphate, of which 50–70% is absorbed, depending on serum phosphate and vitamin D levels. In the first instance, in early CKD, dietary restriction may be sufficient to control plasma phosphate levels. Dietary phosphate is principally in protein-containing foods, and dairy products in particular. Processed foods contain phosphate in significant quantities, as it is a component of moisture and flavour enhancers. Table 1 shows a suggested weight-related daily dietary phosphate intake for children with CKD [22]. Because restriction of dietary phosphate has its principal effects after meals, there may be no change in the morning plasma phosphate levels, so plasma values obtained in the afternoon are more useful in monitoring the effect of phosphate restriction or phosphate binders. The problem with dietary intervention is that foods high in phosphate are also usually high in calcium and vitamin D, so that nutritional 25-hydroxyvitamin D [25(OH)D] and calcium deficiency is common in patients with CKD.
The need for phosphate binders
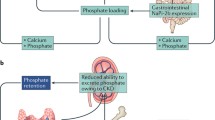
In early CKD, a prescribed decrease in dietary phosphate load reduces FGF-23 levels so that 1,25(OH)2D production and therefore calcium absorption increases and PTH is suppressed. However, the development of resistance to FGF-23 and the increased intestinal absorption of phosphate as a result of increased 1,25(OH)2D means that as CKD progresses, phosphate binders are usually required (Fig. 3). If patients are prescribed vitamin D therapy, absorption of phosphate in the diet increases even further, to up to 85%.
Dialysis
Phosphate is a particular problem for patients on conventional thrice-weekly HD because it is poorly removed by the dialysis process: most is removed in the first hour, and as the rate of movement out of cells is slow, little is removed when the normal range for phosphate is reached. By 12 h post-HD, levels are 80% of predialysis values. Peritoneal dialysis (PD) is equally inadequate at phosphate removal. It has been estimated that approximately 800 mg of phosphate are removed in a standard adult HD session (i.e. 2,400 mg per week) and 300 mg per day in adults on PD (i.e. 2,100 mg/week). In a diet containing ∼1,000 mg of phosphate each day, ∼600 mg would be absorbed (and 400 mg would be excreted in the stool), requiring this amount to be bound or cleared by dialysis. Given the limited clearance by dialysis, there is therefore a need to prevent the absorption of around 300 mg of phosphate per day. Patients on dialysis are the group in whom calcium-containing phosphate binders can cause the most problems with hypercalcaemia because of the reduced ability to excrete calcium in the urine. Use of calcium neutral dialysate (1.25 mmol/l) allows for prescription of larger doses of calcium. Short daily or slow nocturnal HD is the most effective procedure for removing phosphate, to the point that some patients need phosphate supplementation.
Phosphate binders
The ideal phosphate binder would be one that is palatable, effective (i.e. has a high affinity for phosphate therefore necessitating a small tablet load) is not absorbed, is without side effects, can be administered down a feeding tube, is long acting and is inexpensive. No phosphate binder currently available fulfils these criteria.
Phosphate binders are usually divided into calcium containing and noncalcium containing. Calcium-containing preparations have been used the longest but have fallen out of favour because of their theoretical link with soft-tissue calcification; the fear of ectopic calcification with excess calcium intake has led to a switch to newer, non-calcium-containing drugs. Currently, there is no calcium-free phosphate binder licensed for use in children. Phosphate binders must be given with food and must not be given at the same time as iron preparations, as they form insoluble compounds in the gut.
Preparations and cost of commonly used phosphate binders are shown in Table 2. The cost differences of these medications are substantial. For example, three Calcichew 1.25 g per day cost 21p and therefore £76.65 per year. Three sevelamer 800 mg per day cost £2.04 and therefore £744.60 per year. So, for 100 patients, the extra costs for a year for this low-dose of sevelamer would be approaching £70,000 more.
Calcium-containing phosphate binders
The calcium-containing phosphate binders calcium carbonate and calcium acetate are cheaper than non-calcium-containing preparations (Table 2). Calcium carbonate is available in many different formulations, ranging from small, dispersible tablets to large, chewable ones. It causes the least clinical symptoms of all the binders, although gastrointestinal symptoms may occur. Calcium acetate tablets are large and need to be swallowed, although they can be ground up and added to feeds. Gastrointestinal side effects are more severe than with calcium carbonate.
Dissociation of calcium carbonate is maximal below a pH of 5, whereas maximal binding of calcium to phosphate is at a higher pH. It is not, therefore, as effective when given with H2 blockers or proton-pump inhibitors. Calcium acetate, however, has better solubility over a wider range of pH. This means that calcium acetate has a greater binding capacity for the same elemental calcium content so that less calcium is absorbed. Table 3 gives a summary of studies that have looked at calcium absorption and phosphate binding [23]. It might be expected that, for similar phosphate binding, hypercalcaemia would be less common with calcium acetate than with calcium carbonate, but this has not been shown in all studies [24]. Calcium absorption is greater if the binder is taken between meals, when it acts as a calcium supplement. Absorption will also vary with plasma 1,25(OH)2D levels, being as low as 3% in deficiency to presumably higher than the expected normal range in patients who are prescribed activated vitamin D, when hypercalcaemia may occur.
The main issue with calcium-containing binders is the risk of absorption of calcium that cannot be excreted if urine production is reduced, resulting in hypercalcaemia and ectopic calcification (see below).
Non-calcium-containing phosphate binders
Magnesium carbonate
Magnesium carbonate is not commonly used due to its propensity to cause diarrhoea. It is less effective than calcium salts and may be a problem for children on dialysis, who are often already hypermagnesemic [25].
Sevelamer hydrochloride
Sevelamer is a nonabsorbable polymer of allylamine hydrochloride, which functions best at pH 6–7 in the small intestine. It acts like an exchange resin; organic anions, and in particular phosphate, bind to cationic amine groups, displacing the chloride moiety. For this reason an associated metabolic acidosis is common. Sevelamer is now available as the carbonate, which may remove this side effect. As well as phosphate, sevelamer also binds bile salts, thereby exerting a beneficial effect on plasma total and low-density cholesterol [26]. On the other hand, sevelamer also binds fat-soluble vitamins. The tablets need to be swallowed and are difficult to use in children who are dependent on tube feeds because they form a gel that swells inside the tube causing tube blockage. As with all other phosphate binders, it, too, has gastrointestinal side effects.
The first report of the use of sevelamer in children was in 2003 [27]. As would be expected for a calcium-free binder, hypercalcaemia is less common. In a randomized, crossover trial of 8 weeks of treatment with sevelamer and calcium acetate in 18 children with CKD, phosphate control was similar but with less episodes of hypercalcaemia in the sevelamer group, although acidosis was more common [28]. In two studies from the same centre of children on PD with bone-biopsy-proven secondary hyperparathyroidism who were also taking vitamin D and who were randomly assigned to calcium carbonate or sevelamer for 8 months, biochemical and histological abnormalities improved in both groups, but serum calcium levels were at the lower limit of normal in the sevelamer group. The authors suggest that sevelamer, therefore, increases the safety of treatment with activated vitamin D in patients with secondary hyperparathyroidism [29, 30]. On the other hand, 20% of the sevelamer-treated group needed calcium supplements [29], and the development of hypocalcaemia is as high as 24% in adult studies [31].
There is evidence that sevelamer can attenuate the progress of coronary and aortic calcification when compared with calcium-based phosphate binders, either by increasing bone turnover and/or through its effects on lipid, as demonstrated by a study over the course of a year in 200 patients on HD [32]. However, a study of coronary artery calcification progression in 48 children over a 2-year period with CKD stage 5 was not able to demonstrate such a link [33]. The Renagel in New to Dialysis patients (RIND) study also demonstrated that progression of calcification was reduced in 72 patients starting dialysis with sevelamer rather than calcium carbonate, but in 37 patients without calcification at the start, there was no development of calcification over an 18-month period regardless of the binder [34]. In the paediatric study also, there was no new calcification over the 2-year period in those without it at the start [33]. The presence of calcification at the start of the study in adult patients was related to subsequent mortality [32]. However, as yet, the benefits of sevelamer have not been shown to be translatable into a demonstrable improvement in mortality rate. Although the all-cause (i.e. not just cardiovascular) mortality rate in the sevelamer group in the RIND study was borderline better at 44 months (p = 0.05), the study finished at 18 months and binder use after that was dependent on physician’s choice [35]. The Dialysis Clinical Outcomes Revisited trial found no difference in the mortality rate at 2 years in slightly more than 2,000 adult HD patients randomised to either sevelamer or calcium-based binders, being 26% and 27%, respectively, despite the additional lipid-lowering benefits of sevelamer [36].
Sevelamer is of benefit in patients who have a high dietary calcium intake. However, children on a low-phosphate diet who are not receiving a calcium-containing phosphate binder probably do not have a positive calcium balance when they are on maintenance dialysis. Indeed, the Kidney Disease Outcomes Quality Initiative (KDOQI) recommends that in children on sevelamer exclusively, a higher dialysate calcium concentration and/or calcium supplementation with a calcium-containing phosphate binder is used [37]. A study in adults of sevelamer with and without calcium supplements concluded that they were similarly effective and safe in the treatment of end-stage renal disease (ESRD)-related hyperphosphataemia, but that calcium or metabolites of vitamin D with sevelamer may enhance control of hyperparathyroidism [38].
Lanthanum carbonate
Lanthanum is a trivalent cation that binds phosphate ionically and is active over a wide pH. Concerns centre around its safety because of its potential for accumulation in the body, particularly in bone and liver, but absorption is low; <0.0013% is absorbed and is then excreted in bile. Studies in adults show that binding is similar to other calcium binders and there is less hypercalcaemia and a similar incidence of hypocalcaemia to sevelamer. Lanthanum has now been used for 6 years with no evidence of significant side effects [31]. There are no studies in children.
Chitosan chewing gum
An interesting new approach is the use of chewing gum to remove salivary phosphate between meals as well as using phosphate binders with meals. Chitosan is a natural polymer that binds phosphate. Salivary phosphate levels may be as much as five times higher that plasma levels, and in adults, there is between 350 mg and 400 mg of phosphate available to be bound. This approach has been shown to successfully reduce plasma phosphate levels [39].
What is the case for and against calcium-containing phosphate binders?
Recently, there has been a swing away from calcium-containing phosphate binders towards the routine use of sevelamer. The concern is that with the large doses of calcium from calcium-containing phosphate binders, coupled with decreased urinary excretion of calcium, particularly in the face of low bone turnover, hypercalcaemia will occur and cause soft tissue calcification. Although this is a theoretical possibility, evidence of an improvement in survival in patients taking sevelamer is lacking, and even in the world of adult nephrology, there is debate about the best phosphate binder [40, 41]. One excellent review on the subject concludes that “nephrologists are content to spend ever increasing amounts of money on expensive pharmaceutical agents with little evidence that they will improve survival or quality of life” [31]. Unfortunately, drug companies only have to prove efficacy and safety and do not have to demonstrate improved outcomes to obtain a license for their drugs, so it is not in their interest to sponsor clinical trials that may not be to their benefit.
What is the evidence for calcium-containing phosphate binders in children?
Vascular calcification in paediatric patients is not a new phenomenon and was seen at a time when aluminium hydroxide was the only commonly used phosphate binder. Coronary artery abnormalities were identified at post mortem in 12 children who had been on HD in the 1970s, and soft tissue calcification was present in 60% of autopsies undertaken in 120 children with uraemia, dialysis or renal transplants who died between 1960 and 1983 [42]. Most studies are in adults, but there are dangers in extrapolating adult data to children, in whom there is the added dimension of a need for a positive calcium balance in the growing skeleton. Moreover, most adult studies are performed in older patients in whom the skeleton is no longer able to cope with large calcium loads. It also has to be remembered that not all hypercalcaemia is due to intestinal calcium absorption, and that high bone turnover itself can do this by increasing the efflux of calcium from bone. Indeed, hypercalcaemia is described with both sevelamer and lanthanum, and vascular calcification is seen in adult predialysis CKD patients who are not prescribed any phosphate binder [31].
What is the evidence against calcium-containing phosphate binders in children?
Four paediatric studies [16–19, 43] and one in young adults [44] identified a relationship between calcium-containing phosphate-binder intake and cardiac calcification, LVM and/or CIMT. One study was unable to identify any difference in CIMT, PWV or CAC between those taking calcium-containing binders or sevelamer in 85 children on dialysis, but there was a weak correlation between elemental calcium intake and CIMT [15].
Calcium requirements in children are so different to that of adults that adult studies should not be extrapolated to children (see below). However, it must be remembered that the serum calcium level does not reflect the total body calcium load. As free (ionised) calcium in the normal range is crucial to myocardial contractility and several enzymatic reactions, it is tightly regulated and maintained within a narrow range. To support this, the Treat To Goal study showed that the calcium-treated group had virtually identical serum calcium levels compared with the sevelamer-treated group despite ingesting ∼500 g more elemental calcium during the year, and with intestinal absorption being facilitated by concomitant vitamin D therapy, although they did have significantly more hypercalcaemic episodes than the sevelamer group [32].
Although there are no data suggesting that transient mild hypercalcemia has detrimental effects on morbidity in patients with CKD or on dialysis [31, 34], it is likely that episodes of hypercalcaemia in patients on dialysis, in whom calcification inhibitors are compromised, will worsen vascular calcification, particularly in association with high plasma phosphate [8]. In vitro studies have shown that calcium and phosphate act synergistically to increase calcification: when VSMC are incubated in high phosphate media, even a small increase in the media calcium concentration will significantly increase calcification [8, 11, 12, 45]. The transient increases that inevitably occur in clinical practice may go unrecorded but may exacerbate ectopic calcification, particularly in patients on dialysis, in whom calcification inhibitors are compromised and phosphate levels are likely to be high. There is, therefore, an argument regarding children on dialysis with oliguria to reduce their calcium intake and to use a neutral calcium dialysate. However, most important is to maintain a normal plasma phosphate.
What are the calcium requirements throughout life?
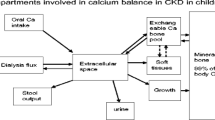
Of the total body calcium, 99% is in the skeleton, 0.6% in soft tissues and 0.1% in extracellular fluid [37]. Total skeletal calcium increases from approximately 25 g at birth to 900 g and 1,200 g in adult women and men, respectively. Calcium balance is therefore positive throughout childhood [46]. At low dietary calcium intakes, a greater proportion is absorbed (approximately 34%) and is proportional to circulating 25(OH)D levels. At higher dietary intakes, there is less influence of vitamin D on calcium absorption and less calcium is absorbed (approximately 29%), i.e. there is adaptation to low 25(OH)D levels and dietary calcium intake [47]. The amount of calcium incorporated into the skeleton increases up to a threshold dietary intake, above which no further bone accumulation occurs. This threshold is influenced by age such that during periods of rapid growth, i.e. infancy and adolescence, calcium balance is at its highest. These high calcium requirements are in comparison with the much lower values in adults (Table 4) [46]. Another factor to remember is the changing normal range for calcium throughout childhood, with high levels at birth that fall thereafter, paralleling the fall in plasma phosphate (Fig. 4).
A low phosphate diet is by definition also low in calcium [and 25(OH)D], and many children may be relying on their phosphate binder to provide adequate calcium intake. Unfortunately, what is not known is how much calcium is absorbed from a dose of calcium-containing phosphate binder. It is likely to vary between individuals, and according to dietary protein, phosphate, fibre and sodium intake and the vitamin D prescription and when it is taken in relationship to food [37, 48]. Studies that have looked at this are summarized in Table 3.
KDOQI gives recommendations for both the minimum and maximum calcium intake, although it admits that these guidelines are opinion based [37, 48]. Logically, minimum recommended intake is 100% of the dietary reference intake (DRI) for calcium, and KDOQI suggests that “the challenge is to ensure that it is achieved”. KDOQI recommends calcium supplementation for infants with CKD who may not get adequate amounts of calcium if not breast fed, if low-electrolyte infant formulas are required or if fluids are restricted; and for children and adolescents on dialysis, in whom a phosphate-restricted diet results in a serious calcium deficit, when typically the dietary calcium intake is <500 mg per day. Calcium-containing phosphate binders may then be the primary source of elemental calcium in the diet [37].
KDOQI also recommends limiting calcium intake from binders and dialysate solutions to prevent soft tissue calcifications as a potential consequence of long-term positive calcium balance, aiming for <2 × DRI or <2,500 mg elemental calcium. It acknowledges, however, that “There are no data on calcium retention as a function of increased long-term calcium intake in patients with CKD, and it is impossible to accurately assess the actual absorption of calcium derived from binders, which is in large part dependent upon the kind and amount of food present in the stomach with the binder” [37]. These calcium intake guidelines have recently been approved by the KDOQI nutrition group [48].
It is possible to roughly calculate the amount of phosphate binder that needs to be prescribed. For example, referring back to Table 3, if 300 mg phosphate needs to be bound per day, then either 7.7 g calcium carbonate (30−31 250-mg tablets or three to four Calcichew Forte per day), 6.7 g calcium acetate (six to seven tablets) or 3.75 g sevelamer (four to five tablets) are required. The elemental calcium content of 7.7 g of calcium carbonate is 3 g, and it is 1.7 g for calcium acetate. If the elemental calcium content of the diet could be expected to be 500 mg, then it is possible to stay within the KDOQI guidelines with calcium acetate but not with calcium carbonate. If only sevelamer is used, then calcium intake will below the DRI. Realistically, many patients do not adhere to a low-phosphate diet so need a combination of a calcium-containing phosphate binder and sevelamer. However, the best way to properly clear phosphate in dialyis patients is for dialysis to be more intensive, such as short hours daily or slow daily nocturnal HD.
Conclusion
So, where does that leave us? The most important factor is dietary assessment of patients’ calcium and phosphate intake, particularly patients on dialysis. It makes no sense to prescribe sevelamer and a calcium supplement, but on the other hand, excess calcium in a child on dialysis is not sensible either. The new KDOQI nutritional guidelines, although opinion based, of minimum intake for calcium of the DRI and maximum of twice the DRI seem appropriate. There does not seem to be any good evidence to not start with calcium-containing phosphate binders, adding sevelamer if the calcium intake is excessive. In the presence of hypercalcaemia, the calcium-binder dose can be reduced or stopped after any prescribed activated vitamin D has been stopped. If hypercalcaemia persists after cessation of calcium binders and vitamin D, then clearly, sevelamer is the binder of choice. As yet, the role of lanthanum carbonate in children is unresearched.
Fortunately, studies relating treatments to mortality cannot be undertaken in children, but it is possible to use well-established surrogate markers of vascular disease. As paediatric nephrologists, we cannot rely on studies from our colleagues in adult nephrology, whose patients have lower calcium requirements and bone accumulation and who often have preexisting vascular disease. We are duty bound to answer for ourselves the very basic, unanswered questions, such as calcium absorption from phosphate binders, the incidence of vascular calcification in children and the role of phosphate control and binder type in its progression.
References
Block GA, Hulbert-Shearon TE, Levin NW, Port FK (1998) Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31:607–617
Prié D, Ureña Torres P, Friedlander G (2009) Latest findings in phosphate homeostasis. Kidney Int 75:882–889
Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M (2008) Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359:584–592
Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW, PREPARE study group (2007) High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22:2909–2916
Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL (2005) Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16:520–528
Goldsmith D, Ritz E, Covic A (2004) Vascular calcification: a stiff challenge for the nephrologist: does preventing bone disease cause arterial disease? Kidney Int 66:1315–1333
Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR (2009) Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20:381–387
Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM (2008) Dialysis triggers VSMC apoptosis, osteo/chodrocytic differentiation and vesicle release to cause accelerated calcification in intact human vessels. Circulation 118:1748–1757
Shroff RC, Shah V, Hiorns MP, Schoppet M, Hofbauer LC, Hawa G, Schurgers LJ, Singhal A, Merryweather I, Brogan P, Shanahan C, Deanfield J, Rees L (2008) The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol Dial Transplant 23:3263–3271
Bellasi A, Kooienga L, Block GA, Veledar E, Spiegel DM, Raggi P (2009) How long is the warranty period for nil or low coronary artery calcium in patients new to hemodialysis? J Nephrol 22:255–262
Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM (2004) Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15:2857–2867
Shroff R, McNair R, Figg N, Skeper J, Deanfield J, Rees L, Shanahan C (2008) Arteries from Paediatric Dialysis Patients Show an Increased Susceptibility to Mineral Ion Induced Apoptosis and Oxidative Stress Leading to Accelerated Vesicle-Mediated Calcification. J Am Soc Nephrol 19:519A
Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM (2000) Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87:E10–E17
Giachelli CM (2009) The emerging role of phosphate in vascular calcification. Kidney Int 75:890–897
Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield J, Rees L (2007) Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18:2996–3003
Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR (2005) Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: role of calcium-phosphorus metabolism. J Am Soc Nephrol 16:2796–2803
Ziolkowska H, Brzewski M, Roszkowska-Blaim M (2008) Determinants of the intima-media thickness in children and adolescents with chronic kidney disease. Pediatr Nephrol 23:805–811
Civilibal M, Caliskan S, Adaletli I, Oflaz H, Sever L, Candan C, Canpolat N, Kasapcopur O, Kuruoglu S, Arisoy N (2006) Coronary artery calcifications in children with end-stage renal disease. Pediatr Nephrol 21:1426–1433
Litwin M, Wühl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Tröger J, Mehls O, Schaefer F (2005) Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16:1494–1500
Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA (2009) Serum Phosphorus Levels Associate with Coronary Atherosclerosis in Young Adults. J Am Soc Nephrol 20:397–404
Isakova T, Gutiérrez OM, Chang Y, Shah A, Tamez H, Smith K, Thadhani R, Wolf M (2009) Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20:388–396
Rees L, Shaw V (2007) Nutrition in children with CRF and on dialysis. Pediatr Nephrol 22:1689–1702
Salusky IB (2006) A new era in phosphate binder therapy; What are the options? Kidney Int 70:S10–S15
Wallot M, Bonzel KE, Winter A, Geörger B, Lettgen B, Bald M (1996) Calcium acetate versus calcium carbonate as oral phosphate binder in pediatric and adolescent hemodialysis patients. Pediatr Nephrol 10:625–630
Delmez JA, Kelber J, Norword KY, Giles KS, Slatopolsky E (1996) Magnesium carbonate as a phosphorus binder: a prospective, controlled, crossover study. Kidney Int 49:163–167
Garland JS, Ross Morton A (2006) Sevelamer hydrochloride in peritoneal dialysis patients: Ca’canny but ca’ awa. Perit Dial Int 26:300–305
Mahdavi H, Kuizon BD, Gales B, Wang HJ, Elashoff RM, Salusky IB (2003) Sevelamer hydrochloride: an effective phosphate binder in dialyzed children. Pediatr Nephrol 18:1260–1264
Pieper AK, Haffner D, Hoppe B, Dittrich K, Offner G, Bonzel KE, John U, Fründ S, Klaus G, Stübinger A, Düker G, Querfeld U (2006) A randomized crossover trial comparing sevelamer with calcium acetate in children with CKD. Am J Kidney Dis 47:625–635
Salusky IB, Goodman WG, Sahney S, Gales B, Perilloux A, Wang HJ, Elashoff RM, Jüppner H (2005) Sevelamer controls parathyroid hormone-induced bone disease as efficiently as calcium carbonate without increasing serum calcium levels during therapy with active vitamin D sterols. J Am Soc Nephrol 16:2501–2508
Wesseling-Perry K, Harkins GC, Wang HJ, Sahney S, Gales B, Elashoff RM, Jüppner H, Salusky IB (2009) Response of different PTH assays to therapy with sevelamer or CaCO3 and active vitamin D sterols. Pediatr Nephrol 24:1355–1361
Hutchison AJ (2009) Oral phosphate binders. Kidney Int 75:906–914
Chertow GM, Burke SK, Raggi P, Treat to Goal Working Group (2002) Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62:245–252
Civilibal M, Caliskan S, Kurugoglu S, Candan C, Canpolat N, Sever L, Kasapcopur O, Arisoy N (2009) Progression of coronary calcification in pediatric chronic kidney disease stage 5. Pediatr Nephrol 24:555–563
Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P (2005) Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68:1815–1824
Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM (2007) Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 71:438–441
Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK (2007) Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 72:1130–1137
National Kidney Foundation (KDOQI) (2005) KDOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis 46(Suppl 1):S1–S122
Chertow GM, Dillon M, Burke SK, Steg M, Bleyer AJ, Garrett BN, Domoto DT, Wilkes BM, Wombolt DG, Slatopolsky E (1999) A randomized trial of sevelamer hydrochloride (RenaGel) with and without supplemental calcium. Strategies for the control of hyperphosphatemia and hyperparathyroidism in hemodialysis patients. Clin Nephrol 51:18–26
Savica V, Calò LA, Monardo P, Davis PA, Granata A, Santoro D, Savica R, Musolino R, Comelli MC, Bellinghieri G (2009) Salivary phosphate-binding chewing gum reduces hyperphosphatemia in dialysis patients. J Am Soc Nephrol 20:639–644
Friedman EA (2006) Calcium-based phosphate binders are appropriate in chronic renal failure. Clin J Am Soc Nephrol 1:704–709
Moe SM, Chertow GM (2006) The case against calcium-based phosphate binders. Clin J Am Soc Nephrol 1:697–703
Milliner DS, Zinsmeister AR, Lieberman E, Landing B (1990) Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int 38:931–936
Shroff R, Egerton M, Bridel M, Shah V, Donald AE, Cole TJ, Hiorns MP, Deanfield JE, Rees L (2008) A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol 19:1239–1246
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342:1478–1483
Shroff RC, Shanahan CM (2007) The vascular biology of calcification. Semin Dial 20:103–109
Matkovic V, Heaney RP (1992) Calcium balance during human growth: evidence for threshold behaviour. Am J Clin Nutr 55:992–996
Abrams SA, Hicks PD, Hawthorne KM (2009) Higher serum 25-hydroxyvitamin D levels in school-age children are inconsistently associated with increased calcium absorption. J Clin Endocrinol Metab 94:2421–2427
KDOQI Work Group (2009) KDOQI Clinical Practice Guideline for Nutrition in Children with Chronic Kidney Disease: 2008 update. Executive Summary. Am J Kidney Dis 53(3 Suppl 2):S11–104
Clayton BE, Jenkins P, Round JM (1980) Paediatric chemical pathology: clinical tests and references ranges. Oxford: Blackwell Scientific
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rees, L., Shroff, R.C. Phosphate binders in CKD: chalking out the differences. Pediatr Nephrol 25, 385–394 (2010). https://doi.org/10.1007/s00467-009-1329-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-009-1329-0