Abstract
Most patients with idiopathic nephrotic syndrome are steroid-responsive, but about 50% relapse and often become steroid-dependent and exposed to long-term steroid complications. The aim of this study was to determine predictive risk factors for steroid and/or cyclosporine A (CyA) dependence. In France, steroid responsiveness is defined as remission after 1 month of oral prednisone (60 mg/m2 per day) and—in the case of persistent proteinuria on day 30—three methylprednisolone pulses (MPP; 1 g/1.73 m2 on days 1, 3, and 5). Thirty-five steroid-responsive children, followed between 1999 and 2006, were included in this study. Median age at diagnosis was 4.9 years. All patients initially received prednisone 60 mg/m2 per day. Twenty-four of the 35 patients were steroid-dependent, with 12 requiring MPP. Of the latter 12 patients, 83.3% were treated with CyA during follow-up; in comparison, only 16.7% of the patients who did not receive MPP required CyA during follow-up (chi-square test, P = 0.001). T risk for steroid dependence was 100% in our cohort if remission was achieved after day 20. Patients who need MPP are at high risk to require CyA to achieve disease control. By identifying these children, we could use adequate immunosuppressive drugs earlier and reduce morbidity related to steroids and multiple relapses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic nephrotic syndrome (INS) is the most frequent glomerular disease in childhood, with an annual incidence of approximately two to three new cases per 100,000 population under the age of 18 years [1, 2]. The age at onset is generally between 18 months and 6 years, and there is a 2:1 male/female ratio. The International Study of Kidney Disease in Children guidelines for the initial treatment of patients between 18 months and 6 years presenting with INS are based upon a high-dose prednisone therapy (60 mg/m2 daily) for 4 weeks. Most of patients are steroid-responsive, going into complete remission, but about 50% relapse and often become steroid-dependent. In France, steroid sensitivity is defined as remission induced by oral prednisone (60 mg/m2 per day) over 30 days, followed by three methylprednisolone pulses (MPP) of 1 g/1.73 m2 every other day (treatment days 31, 33, and 35) in the case of nephrotic proteinuria by treatment day 30 [3].
The prolonged administration of continuous or discontinuous steroids can be associated with long-term complications, such as short stature, susceptibility to infections, obesity, hypertension and hirsutism [2]. Steroid-sparing agents, including levamisole, cyclophosphamide, mycophenolate mofetil, cyclosporine A (CyA) or, more rarely, tacrolimus [4, 5] and rituximab [6], are used to reduce or avoid steroid toxicity.
The aim of this study was to determine predictive risk factors for the development of high-degree steroid dependency early in the disease course. A stepwise increase of immunosuppression is often related to multiple relapses and an increase of the cumulative steroid dose. The early identification of those patients who require CyA for disease control could reduce the number of relapses and the cumulative steroid dose and, thereby, steroid- and disease-related morbidity.
Patients and methods
Patients
All children who had been referred with a first manifestation of INS to the department of pediatric nephrology of the Armand-Trousseau hospital, Paris, between 1999 and 2006 were considered for inclusion in this retrospective study. These patients were identified with INS as a discharge code or a text word in their computerized hospital records. Children with a follow-up <12 months were not included, and steroid-resistant patients were also not included.
A relapse was defined by a urine dipstick reaction ≥3+ protein for 3 consecutive days associated to a decrease of serum albumin to <30 g/l. Complete remission was defined by a persistent negative or trace urine dipstick reaction, confirmed by measurement of urinary protein und creatinine. An initial remission followed by two or more relapses during the steroid reduction period or within 1 month after tapering off prednisone was defined as steroid dependence. Steroid resistance was defined by persistent proteinuria after 30 days of oral prednisone (60 mg/m2) and 3 doses of MPP (1 g/1.73 m2) on days 31, 33 and 35.
All patients initially received prednisone (60 mg/m2 per day) for 30 days. The relapses were treated by oral prednisone at 60 mg/m2 per day until remission plus 7 additional days, then the prednisone dose was tapered to 60 mg/m2 every other day (EOD) for 4 weeks, followed by a reduction of 15 mg/m2 EOD per week to the lowest dose necessary to maintain remission and, ultimately, discontinuation of treatment if appropriate. If the patient was not in remission after 1 month of oral prednisone, three doses of MPP (1 g/1.73 m2 EOD) were given. Those patients who went into remission after MPP administration were continued on oral prednisone at 60 mg per m2 every other day for 2 months and then tapered every 2 weeks in the usual manner and discontinued if appropriate. Only patients who did not respond to MPP were considered here to be steroid resistant. They were not included in this study.
Steroid-sparing agents were introduced if the patient presented frequent relapses (>2 relapses per year), and/or if the steroid dosage to maintain remission was ≥20 mg/m2 EOD and/or if steroid related side effects were unacceptable in terms of growth retardation or obesity despite adequate diet. The initial steroid-sparing agent used was levamisole; if relapses occurred under levamisole while prednisone was ≥20 mg/m2 EOD, we introduced oral cyclophosphamide (cumulative dose of 170 mg/kg over 3 months); if the patient relapsed while on prednisone ≥20 mg/kg EOD, we introduced mycophenolate mofetil (MMF). If the patient relapsed on MMF while the prednisone dose was ≥20 mg/m2 EOD, oral CyA was introduced. The initial CyA dose was 5 mg/kg daily and was adjusted to maintain blood CyA at levels between 100 and 150 ng/mL. Patients who relapsed after cyclophosphamide treatment before 2003 received CyA, as experience with MMF was limited at this time. The concept of a stepwise increase of immunosuppressive therapy was applied for the vast majority of patients. The exceptions were those patients with a very high degree of steroid dependancy with relapses under prednisone at 60 mg/m2 EOD. In these patients, the first steroid-sparing agent was cyclophosphamide instead of levamisol. For all patients, cyclophosphamide was given before the introduction of CyA. Treatment for all 24 patients is summarized in Table 1. Non-effectiveness of cyclophosphamide was defined as relapse while prednisone was ≥20 mg/m2 EOD.
All these treatments were individually adapted according to patient’s history and adverse effects due to immunosuppressive treatment.
Statistical analyses
Age at onset, gender, days to remission after initial steroid therapy, MPP, number of relapses, mean steroid dose, immunosuppressive drugs, serum creatinine and steroid-related adverse events were collected and considered as potential predictive factors of CyA requirement.
Statistical analysis were performed using Sigma Stat ver. 3.5 (Systat, San Jose, CA) or EPIINFO EPI INFO software (Centers for Disease Control and Prevention, Atlanta, GA), and graphs were created using Sigma Plot ver. (Systat). We first described the main clinical and biological characteristics of the population. Then, for the first analyses, the requirement to MPP was considered as the outcome. We studied the relationship between the requirement to MPP and age, gender, number of days to achieve remission after initial steroid therapy, number of relapses and steroid dependency, respectively. For dichotomous variables, we calculated an odds ratio (OR), its 95% confidence interval (CI) using the maximum likelihood method and a Fischer exact test. If the 2×2 table contained a zero cell, we calculated a corrected OR by adding 0.5 to each cell of the table [7]. The distributions of continuous variables were compared using the non parametric Mann–Whitney test because of non-homogeneity of variance. For further analyses, we considered the CyA requirement to control the disease course as the outcome to be predicted. We studied the relationship between CyA requirement to maintain remission and all the previous variables, including the requirement to MPP. Finally, we evaluated the discriminating power of MPP for CyA predictability, calculating sensibility, specificity, positive and negative predictive values, and positive and negative likelihood ratios (LR).
Results
Patients characteristics
Thirty-five patients (25 boys, two Africans, 33 Caucasians) were included. They were diagnosed with INS at a mean age of 4.9 years (SD 3.6, range 1.5–16 years). Among the 35 steroid-sensitive patients, 24 (69%) became steroid-dependent, and 11 (31%) were not steroid-dependent and did not require MPP or steroid-sparing agents. Twelve (50%) of the steroid-dependent patients required MPP to obtain remission. The mean number of relapses was 3.5 (SD 3.9, range 0–19), and the mean follow-up was 4.2 years (SD 1.6). Among the steroid-dependant patients, the mean dose of steroid therapy required was 1.1 mg/kg per day (SD 1.1, range 0.3–3.0 mg/kg per day). Immunosuppressive drugs used to control disease in the 24 (69%) steroid-dependent patients were: levamisol (14 patients, 40%), cyclophophamide (19 patients, 79%), MMF (9, 38%), and CyA (12, 34%). The mean number of relapses before the use of CyA was 3.8 (SD 1.9, range 1–8).
Adverse effects associated with steroid therapy were hypertension (normalized under amlodipin; 0.15 mg/kg twice daily) in three patients from the group who received MPP and growth retardation for one patient. None developed infectious complications or avascular bone necrosis. Adverse effects associated with CyA therapy were infrequent and reversible: CyA was switched to tacrolimus in one patient because of severe hypertrichosis. The duration of CyA treatment was 19 (range 2–131) months. Four patients were biopsied because CyA treatment duration exceeded 24 months. All biopsies revealed minimal change disease without histological evidence for CyA nephrotoxicity. One case of reversible acute renal failure was noted, but there was no case of chronic renal failure.
MPP requirement
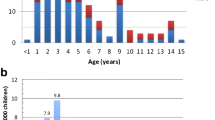
The distribution according to the time interval between the first day on oral prednisone and remission is displayed in Fig. 1. Among the children who had not achieved remission by the 20th day of initial steroid treatment, 100% required MPP and became steroid-dependent. None of the other variables (age at disease onset, sex, number of days to remission at first disease manifestation, level of steroid dependency or number of relapses) were significantly related to MPP requirement. A comparison of steroid-dependent patients who required MPP and those who did not is given in Table 2.
Distribution of steroid-responsive patients without steroid dependency (open block), steroid-dependent patients without methylprednisolone pulses (MPP) (hatched block) and steroid-dependent patients who required MPP (diagonal striped block) according to the rapidity of remission during the initial prednisone course
Cyclosporine A
Twelve steroid-dependent patients required CyA. The time interval between disease onset and introduction of CyA was 14.1 months (range 2–37 months), regardless of the need for MPP.
The median number of days to achieve remission after the first steroid therapy was significantly higher in children who required CyA than in those who did not: 20.0 vs. 8.0 days, respectively (P = 0.02). Of the children who had not achieved remission by day 20 of the initial prednisone treatment, 100% required CyA. There was a strong and statistically significant association between CyA requirement and MPP treatment (OR 52.5, 95% CI 5.0–694, P < 0.001). The steroid dependency was also significantly associated to MPP requirement (Table 2). None of the other variables were significantly related to CyA requirement.
Among the 12 patients with CyA requirement, ten had previously received MPP to achieve remission. The requirement for MPP had a sensitivity of 91% (95% CI 59–100), and a specificity of 85% (95% CI 55–98). The requirement for MPP also had positive and negative predictive values for CyA requirement of 83 (95% CI 52–98) and 92% (95% CI 62–100), respectively; the positive and negative likelihood ratios were 5.9 (95% CI 1.6–21) and 0.1 (95% CI 0.01–0.7).
Discussion
Identification of the character of INS during the first disease manifestation is challenging. We have demonstrated that nephrotic children who require MPP to achieve remission during the first manifestation are at high risk to require CyA for disease control. Further, in our cohort, all of those patients who did not achieve remission by day 20 of the initial prednisone course required MPP.
Efforts have been made earlier to identify the relapse pattern of a child presenting with a first manifestation of INS [8, 9]. These researchers found that the presence of microscopic hematuria and/or remission after day 9 of the initial prednisone treatment were predictive factors for steroid dependency.
Steroid dependency is a frequent risk for children with INS. Discontinued steroid usage may decrease the risks for steroid toxicity, but patients with frequently relapsing nephrotic syndrome may require prednisone doses above the cut-off level for steroid toxicity.
It is well known that disease activity in INS has a natural tendency to decrease over time in most cases [10]. Disease control may, therefore, require a more intensive immunosuppression at disease onset. For steroid-dependent patients, pediatric nephrologists have to make a choice between a steroid-based treatment with the known adverse effects and immunosuppressive drugs with a risk of over-immunosuppression and drug-specific side effects, such as calcineurin inhibitor-related nephrotoxicity. Predictive factors for high-degree steroid-dependent forms of INS may therefore be of interest and provide some therapeutic guidelines for the attending physician(s).
Our results suggest that high-degree steroid dependency is very probable if the patient had required MPP to obtain remission at disease onset. The risk of overestimating steroid dependency seems to be relatively low, but it has to be taken into consideration. Such an overestimation of steroid dependency would lead to unjustified use of oral CyA. In our study, two of 12 children did not require CyA, although they required MPP to obtain remission. In most countries, patients with persistent nephrotic proteinuria after a course of high-dose oral prednisone are considered to be steroid-resistant and are mostly treated with oral CyA [11, 12]. However, these treatment protocols are often based on a 6-week prednisone course at disease onset and/or a maximum daily dosage of 80 mg. One may hypothesize that some of the patients who were considered to be high-degree steroid-dependent in our study would probably be considered as steroid-resistant in several other countries.
The consideration of a patient as steroid-resistant automatically implies the use of CyA, which is still the only validated treatment option for steroid-resistant nephrotic syndrome [13]. The treatment strategy in France is such that as many patients as possible should not to be started on CyA in an effort to avoid nephrotoxicity. However, in our study we have shown that the vast majority of patients (83%) who require MPP ultimately also require CyA to maintain remission. Therefore, the question of whether MPP really helps is a justifiable one.
In general, after the use of MPP, patients either obtain remission within several days or fail to respond completely. Therefore, the potential benefit of MPP on the short term is earlier remission for certain patients. On the long term, the benefit of MPP is to individualize the therapeutic strategy by defining disease activity as precisely as possible. The administration of MPP after oral prednisone helps define two groups of patients who are resistant to 1 month of oral prednisone: those who respond to MPP and those who do not. The first group may be considered as intermediate, and the treatment strategy could be adapted accordingly. Future studies are necessary to answer the question if such patients who go into remission after MPP may benefit from a less aggressive management (e.g. shorter CyA treatment with a lower dosage and/or early switch to MMF). For the others (those who do not respond to MPP), a more aggressive treatment strategy may be necessary. Treatment with MPP seems to facilitate adaptations and/or individualization of the therapeutic approach of the attending physicians.
In our cohort, those children who went into remission after day 20 of the initial prednisone course all required MPP and became steroid-dependent. At first glance, this might suggest that the treatment duration of the initial prednisone course could have been reduced to 20 days. However, higher cumulative doses in the initial prednisone course have been shown to be associated with a more favorable disease course on the short and long term [14]. It would seem to be beneficial to maintain at least a 1-month prednisone course at disease onset for all patients.
Those children who required MPP to obtain remission went through several immunosuppressive treatments during the disease course, with a stepwise increase of immunosuppression. The aim of immunosuppressive drugs was to taper steroids to a non-toxic level of <15 mg/m2 EOD without disease relapse. In those ten children who required MPP, there were a total of 60 relapses between achievement of the first remission after MPP and introduction of CyA. As each relapse is associated with disease-related thrombembolic or infectious risks and an increase of steroid dosage, earlier adequate immunosuppression with stable disease control should reduce disease morbidity. Further, longer periods of high-range proteinuria due to uncontrolled INS may be nephrotoxic and result in interstitial fibrosis. Apart from adequate immunosuppression and disease control, the use of CyA may have beneficial effects on disease course: Hoyer et al. have shown recently that an 8-week course of oral CyA at disease onset significantly reduced the relapse rate in the first 9 months following disease onset [15].
The risk of unjustified use of CyA for some patients may cause some hesitation. Recent data revealed an increased risk for CyA-related nephrotoxicity if treatment duration exceeds 36 months. These data also suggest that CyA treatment should be avoided if possible in children younger than 5 years [16]. As long as the treatment duration of oral CyA is kept short (<12 months) and the dosage is ≤5 mg/kg per day, there is currently no evidence of irreversible renal damage [17] on control biopsies. Nephrotoxicity from CyA treatment is less studied in INS than in transplantation, but it seems to be less severe, especially if treatment duration is short [18, 19]. However, it is well known that the most severe cases of steroid-dependent NS frequently occur in children in the first 5 years of life [20]. Therefore, one has to carefully analyze the benefit risk ratio of CyA treatment in such patients. Moreover, CyA nephrotoxicity is at least partially reversible after a short period of oral CyA treatment, as its major pathophysiological element seems to be prolonged vasoconstriction and not necessarily interstitial fibrosis and arteriolar hyalinosis [21].
The use of MMF instead of CyA in patients who have required MPP should be mentioned, although since our patient cohort consisted of patients with a first disease manifestation from 1999 to 2006, we can report little experience on the use of MMF in steroid-dependent patients. More data are currently available about the effects of MMF in high-degree steroid dependency [22–25] so that an early switch from CyA to MMF [22, 26, 27] and/or a switch from MMF to CyA in the case of relapse could be included in the discussion of future protocols.
In conclusion, patients who require MPP to obtain remission during first disease manifestation are at risk of requiring CyA for disease control. These patients are a subgroup of all patients who show resistance to a 1-month course of oral prednisone. By identifying these children, we could eventually use adequate immunosuppressive drugs earlier and reduce morbidity related to steroids and multiple relapses.
Abbreviations
- CyA:
-
Cyclosporine A
- EOD:
-
Every other day
- INS:
-
Idiopathic nephrotic syndrome
- MMF:
-
Mycophenolate mofetil
- MPP:
-
Methylprednisolone pulses
References
Filler G, Young E, Geier P, Carpenter B, Drukker A, Feber J (2003) Is there really an increase in non-minimal change nephrotic syndrome in children? Am J Kidney Dis 42:1107–1113
Eddy AA, Symons JM (2003) Nephrotic syndrome in childhood. Lancet 362:629–639
Murnaghan K, Vasmant D, Bensman A (1984) Pulse methylprednisolone therapy in severe idiopathic childhood nephrotic syndrome. Acta Paediatr Scand 73:733–739
Sinha MD, MacLeod R, Rigby E, Clark AG (2006) Treatment of severe steroid-dependent nephrotic syndrome (SDNS) in children with tacrolimus. Nephrol Dial Transplant 21:1848–1854
Loeffler K, Gowrishankar M, Yiu V (2004) Tacrolimus therapy in pediatric patients with treatment-resistant nephrotic syndrome. Pediatr Nephrol 19:281–287
Benz K, Dotsch J, Rascher W, Stachel D (2004) Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol 19:794–797
Deeks JJ, Higgins JPT, Altman DG (2004) Analysing and presenting results. In: Alderson P, Green S, Higgins JPT (eds) Cochrane reviewers' handbook 422 (updated March 2004). John Wiley & Sons, Chichester, pp 68–139
Yap HK, Han EJ, Heng CK, Gong WK (2001) Risk factors for steroid dependency in children with idiopathic nephrotic syndrome. Pediatr Nephrol 16:1049–1052
Constantinescu AR, Shah HB, Foote EF, Weiss LS (2000) Predicting first-year relapses in children with nephrotic syndrome. Pediatrics 105:492–495
Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS (1985) Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1:368–370
Mori K, Honda M, Ikeda M (2004) Efficacy of methylprednisolone pulse therapy in steroid-resistant nephrotic syndrome. Pediatr Nephrol 19:1232–1236
Yorgin PD, Krasher J, Al-Uzri AY (2001) Pulse methylprednisolone treatment of idiopathic steroid-resistant nephrotic syndrome. Pediatr Nephrol 16:245–250
Niaudet P, Fuchshuber A, Gagnadoux MF, Habib R, Broyer M (1997) Cyclosporine in the therapy of steroid-resistant idiopathic nephrotic syndrome. Kidney Int Suppl 58:S85–S90
Arbeitsgemeinschaft für Pädiatrische Nephrologie (1988) Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet 1:380–383
Hoyer PF, Brodehl J (2006) Initial treatment of idiopathic nephrotic syndrome in children: prednisone versus prednisone plus cyclosporine A: a prospective, randomized trial. J Am Soc Nephrol 17:1151–1157
Fujinaga S, Kaneko K, Muto T, Ohtomo Y, Murakami H, Yamashiro Y (2006) Independent risk factors for chronic cyclosporine induced nephropathy in children with nephrotic syndrome. Arch Dis Child 91:666–670
Tanaka H, Nakahata T, Ito E (2004) Single-dose daily administration of cyclosporin A for relapsing nephrotic syndrome. Pediatr Nephrol 19:1055–1058
Tejani AT, Butt K, Trachtman H, Suthanthiran M, Rosenthal CJ, Khawar MR (1988) Cyclosporine A induced remission of relapsing nephrotic syndrome in children. Kidney Int 33:729–734
Tejani A, Suthanthiran M, Pomrantz A (1991) A randomized controlled trial of low-dose prednisone and ciclosporin versus high-dose prednisone in nephrotic syndrome of children. Nephron 59:96–99
Fakhouri F, Bocquet N, Taupin P, Presne C, Gagnadoux MF, Landais P, Lesavre P, Chauveau D, Knebelmann B, Broyer M, Grunfeld JP, Niaudet P (2003) Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis 41:550–557
Cattaneo D, Perico N, Gaspari F, Remuzzi G (2004) Nephrotoxic aspects of cyclosporine. Transplant Proc 36:234S–239S
Ulinski T, Dubourg L, Said MH, Parchoux B, Ranchin B, Cochat P (2005) Switch from cyclosporine A to mycophenolate mofetil in nephrotic children. Pediatr Nephrol 20:482–485
Afzal K, Bagga A, Menon S, Hari P, Jordan SC (2007) Treatment with mycophenolate mofetil and prednisolone for steroid-dependent nephrotic syndrome. Pediatr Nephrol 22:2059–2065
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42:1114–1120
Gellermann J, Querfeld U (2004) Frequently relapsing nephrotic syndrome: treatment with mycophenolate mofetil. Pediatr Nephrol 19:101–104
Fujinaga S, Ohtomo Y, Umino D, Takemoto M, Shimizu T, Yamashiro Y, Kaneko K (2007) A prospective study on the use of mycophenolate mofetil in children with cyclosporine-dependent nephrotic syndrome. Pediatr Nephrol 22:71–76
Mendizabal S, Zamora I, Berbel O, Sanahuja MJ, Fuentes J, Simon J (2005) Mycophenolate mofetil in steroid/cyclosporine-dependent/resistant nephrotic syndrome. Pediatr Nephrol 20:914–919
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Letavernier, B., Letavernier, E., Leroy, S. et al. Prediction of high-degree steroid dependency in pediatric idiopathic nephrotic syndrome. Pediatr Nephrol 23, 2221–2226 (2008). https://doi.org/10.1007/s00467-008-0914-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0914-y