Abstract
Impaired renal development during foetal life is a proposed mechanism for adult hypertension in people born small. Whether preterm birth contributes to such adverse development is still unclear. We investigated the selective contributions from foetal growth restriction or preterm birth to renal function and volume in children with low birth weight. Three groups of 9 to 12-year-old children were studied: those born at < 32 gestational weeks (preterm, n = 39), those born at term but small for gestational age (SGA, n = 29) and those born at the term appropriate for gestational age (controls, n = 37). We estimated renal function by calculating glomerular filtration rate (GFR) and by measuring urinary proteins. Volumetric ultrasound of the kidneys was performed in 86 children (preterm, n = 33; SGA, n = 25; controls, n = 29). Estimated glomerular filtration rate (eGFR) and urinary protein patterns were similar between the groups. Kidney volume (preterm 162 ml (31); SGA 163 ml (26) and controls 182 ml (47)) was smaller in the preterm group than in the controls, but the difference was not significant when adjusted for body surface area, gender and age (P = 0.25). Total renal volume correlated to birth weight (r = 0.23, P = 0.03). No significant differences were found in renal function or volume between the three groups at school age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low birth weight is a risk factor for the development of hypertension and cardiovascular disease later in life [1]. The causal pathways for this association are still unclear. Brenner et al. [2] and Brenner and Chertow [3] introduced the theory that poor foetal growth would result in fewer nephrons and increased renal disease later in life. Fewer nephrons, with subsequent hyperfiltration and glomerulosclerosis in the remaining nephrons, could lead to accelerated ageing and early loss of renal function. This hypothesis is supported by human post-mortem and animal studies, where low birth weight is found to correlate to fewer nephrons and, thereby, reduced kidney weight/volume [4–8]. Other studies imply that renal disease in adults can be correlated to low birth weight [9, 10].
Few studies differentiate between low birth weight due to intrauterine growth restriction (IUGR) and that due to prematurity. Very preterm infants have a precarious post-natal period, with inadequate nutrition, poor growth, medical complications and a stressful environment, which could compromise kidney development [11]. We have previously shown that preterm birth is associated with decreased renal function during the first year of life [11], higher catecholamines and heart rates in childhood [12] and higher blood pressure in adolescent and adult women and men [13–15].
We undertook this study to investigate whether premature birth and low birth weight due to IUGR are risk factors for smaller kidneys and renal impairment in childhood.
Subjects and method
Subjects
Three groups of healthy school children with different perinatal exposures were investigated: 39 children born before 32 gestational weeks (preterm), 29 born at term and small for gestational age (SGA), and 37 born at term with appropriate birth weight for gestational age (control). Gestational age was determined by ultrasound in early pregnancy [16]. Term was defined as a gestational age between 37 and 42 weeks. SGA was defined as a birth weight < mean −2 standard deviations (SDs) (i.e. below the gender-specific 2.5th centile for gestational age) according to Swedish reference data for normal foetal growth [17]. Of the 29 children in the SGA group, ten had a ponderal index (PI) < 2.3, suggesting asymmetrical intrauterine growth. PI was calculated as birth weight (in kilogrammes) × 100/birth length (in centimetres, cubed) [18]. In the six children in the preterm group who were also SGA, the PI was similar to that of the other preterm children [mean (SD) 2.15 (0.31) and 2.18 (0.42)].
To compare birth weights in the three groups we used the z-score, calculated by subtracting the individual birth weight from the expected birth weight, according to the Swedish reference curve [17], and dividing that difference by the standard deviation of birth weights in the three groups, respectively. The z-scores in the preterm, SGA and control groups were −0.72 (1.03), −3.14 (0.59) and −0.05 (0.87).
All children were singletons, born in northern Stockholm at Karolinska Hospital, without chromosomal anomalies, congenital infections, life-threatening congenital anomalies or urogenital malformations. The preterm and SGA groups consisted of children born consecutively and cared for at the neonatal intensive care unit (NICU) and the maternity ward, respectively. Subsequently, children born at term with normal birth weights (controls) and matched for date of birth and gender were selected from the delivery room records. All control infants were healthy at birth and had not been admitted to the NICU. A total of 390 children were eligible for the study, but 133 could not be reached due to changed addresses. Of the remaining 257 invited families, 105 accepted the invitation to participate in the study. The perinatal characteristics of the children not participating were similar to those of the three study groups—the gestational age (weeks) and birth weight (grammes) were 27.5 (2.0) and 987 (243) in the preterm group, 39.5 (1.2) and 2,460 (269) in the SGA group, and 40.1 (1.0) and 3,635 (449) in the controls, respectively.
Information on maternal and neonatal characteristics was collected from the Swedish birth records. Maternal age, maternal smoking and maternal history of hypertension/pre-eclampsia [19], prenatal steroid administration, date of birth, gestational age, birth weight, Apgar scores, and highest serum creatinine value after the first postnatal week of life and highest serum C-reactive protein (CRP) level during stay in the NICU were noted. The medical charts were reviewed for pertinent data, including ventilatory support and neonatal morbidity for the preterm subjects. Maternal, perinatal and current characteristics for the 105 children investigated are presented in Tables 1, 2 and 3.
The study was approved by the Ethics Committee at Karolinska University Hospital. Written consent was obtained from the parents and children.
First study period: determination of renal function and blood pressure
Renal function tests were performed when the children were, on average, 9 years old [12]. Of the 105 children, 21 refused blood sampling. Accordingly, estimated glomerular filtration rate (eGFR), in millilitres per minute per 1.73 m2 body surface area, was calculated in 84 children (28 preterm, 23 SGA and 33 controls) (Table 2). We used serum creatinine level, analysed by the Jaffe method (Beckman Coulters instrument LX, Fullerton, California, USA), to calculate eGFR by the Schwartz formula [20]. Serum cystatin-C was measured with the Dade Behring Cystatin C assay with the automated Dade Behring Nephelometer II (BNII).
In 55 children the preparation of the first morning urine sample was unsuccessful. Urine albumin, urine immunoglobulin G (U-IgG) and urine alpha-1 microglobulin (U-A1M) were analysed by the immunonephelometry method, and urinary N-acetylglucosamine (U-NAG) was analysed by the colorimetric method (Cobas Mira, Hoffmann–La Roche AG, Basel, Switzerland) in 12 preterm, 22 SGA and 16 control children. Urinary protein excretions were calculated as ratios to urinary creatinine, in milligrammes or nanokatals per millimole creatinine. The urinary proteins were used as markers not only of glomerular damage due to hyperfiltration but also for signs of tubular damage due to dysplasia or interstitial fibrosis.
Blood pressure and anthropometric measurements were performed by the same research nurse during a morning visit to Karolinska University Hospital [12]. Briefly, after the children had fasted overnight and rested for at least 15 min, their blood pressures (BPs) were recorded while they were lying down with their arms at heart level. An automated, non-invasive oscillometric technique (Dinamap) [21] was used, and an average of three readings determined the resting BP values. The intra-individual coefficient of variation for resting BP in children has been found to vary between 6% and 9% in our laboratory [13]. Following resting measurements, the subjects were asked to stand up. An average of three consecutive BP measurements obtained 30 s after the subjects had stood up was calculated and designated the standing BP. After the subjects had sat down and resting for at least 15 min, a mathematical stress test was performed after which BP was determined.
Second study period: determination of kidney volume
Kidney volumes were measured when the children had reached an average age of 12 years. At this time, 19/105 declined to participate. The volumes were measured by ultrasound in 86 children (33 preterm, 24 SGA and 29 controls). All investigations were done by the same investigator using the same ultrasound system (Accuson 128XP with three 5.5 MHz linear transducers). The children were examined while in a prone position, and a pillow ventral to the upper abdomen was used to compensate for lumbar lordosis. The kidney was identified in the sagittal plane along the longitudinal axis. In this position, up to three longitudinal measurements of the largest length and width were performed. The probe was than rotated 90° and the measurements of depth and width at the hilar level were taken. The highest measurements were consistently used for calculations. Renal volume was established by the formula for an ellipsoid: length × thickness × width × 0.5233, and calculated in cubic centimetres (cm3) [22]. Left and right kidney volumes were separately investigated and then the values were added together to provide the total kidney volume.
Anthropometric data were obtained for all children by the same research nurse who performed the same measurements for the first study period. Body mass index (BMI) was calculated by the formula: weight (kg)/height (m2). Body surface area (BSA), in square metres, was measured according to the formula from Haycock (0.024265 × height(cm)0.3964 × weight(kg)0.5378) [23].
Statistics
Data are presented as numbers or as mean values with one standard deviation (SD).
Normal probability plots confirmed the normal distribution for the different variables, including renal volume in the three groups. In two control children, total renal volume (300 ml and 340 ml, respectively) exceeded +3 SDs from the mean (169 ml). These observations where considered as outliers and were excluded from further statistical testing. Bartlett’s test did not disclose any significant differences in variances between the three groups (P = 0.59).
Analyses were computed with the STATA software package, version 9, using the analysis of variance (ANOVA) command presenting regression coefficients for the specified models. Reported P values from the ANOVA command were the P values for the regression coefficients for the preterm and term SGA categories. A P value of less than 0.05 was defined as significant. We used analyses of variance to analyse group differences in kidney function and volumes.
For renal volume comparisons, we adjusted for gender, age, and BSA. BSA and age were entered as continuous variables and gender as a categorical variable (boys/girls). Associations between renal volume and weight, length and BSA at 13 years of age—for all children and for the individual groups—were all significant and similar, with the exception of the SGA group where none of the variables was statistically correlated.
Due to the heterogeneous body dimensions in the three groups of children we thought that it would be reasonable to adjust for BMI as well as for BSA (Table 3). We therefore also present the results after adjusting for BMI instead of BSA (Table 4), as supported by other authors [24–26]. It is important to mention that the significant differences between the groups was stronger for BSA than for BMI (P = 0.017 versus P = 0.045), supporting BSA as the better adjustment factor.
We considered group differences in renal function and renal volume of 10% as important. For this purpose we calculated the sample size to 30 in each group, with 80% power and a significance level of 0.05.
Analyses of BP were adjusted for height and maternal pre-eclampsia. Besides being potential confounders, both factors were unequally distributed in the three study groups.
We used regression analyses to study possible associations between kidney function and volume and perinatal factors. Simple logistic regression was used for continuous variables, and the chi-square test was used for binary variables. We used stepwise forward regression, entering independent variables with P < 0.20 in simple regression analyses. Independent variables included: gestational age at birth, birth weight, gender, Apgar score at 10 min, birth weight z-score, maternal hypertension during pregnancy, delivery mode, prenatal and/or postnatal steroid administration, patent ductus arteriosus (PDA) and duration of continuous positive pressure support. Independent variables tested, but not included in the regression analysis, were: age at study, respiratory distress syndrome, bronchopulmonary dysplasia, necrotising enterocolitis and intraventricular haemorrhage.
Results
Kidney function
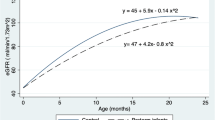
The mean eGFR was 88.8 ml/min per 1.73m2 (12.0) and it did not differ significantly between the three groups (Table 2). Concentrations of serum creatinine and serum cystatin C did not differ between the groups (P = 0.146 and P = 0.113, respectively). In addition, the urinary protein patterns for albumin, NAG, IgG and A1M in relation to urinary creatinine were similar in the three groups (Table 2).
BP results have been presented in a previous publication (Table 2) [12]. There were no differences between the groups. Twelve children (five preterm, three SGA, four controls) had resting blood pressure in the hypertensive range, after adjustment for age and gender, and height-adjusted diastolic or systolic BP ≥ 90th percentile on the study day.
Kidney volume
The total kidney volume was 162 (31) ml in children born preterm, 163 (26) ml in children born SGA and 182 (47) ml in the control group (P = 0.073 between all groups; P = 0.035 preterm versus control; P = 0.069 SGA versus control). Separate values for the right and left kidney volumes are presented in Table 3. After adjustment for BSA and gender, differences in total kidney volume between preterm and control groups became non-significant (7.5 ml lower in preterm, P = 0.255), as also after we had excluded the six subjects born SGA in the preterm group (12.9 ml lower in preterm, P = 0.076, Table 4). In the SGA group, renal volume was not different from that of controls (Table 4).
Birth weight correlated to total renal volume (r = 0.23, P = 0.03). In the preterm children, total renal volume did not correlate to gestational age (r = 0.33, P = 0.06) but it correlated to birth length (r = 0.36, P = 0.04).
After adjusting for, gender and age, and BMI instead of BSA, we found that total kidney volume was 14.3 ml lower in children born preterm than in controls (P = 0.046) (Table 4). The difference increased to a 19.9 ml lower kidney volume in preterm children compared with controls after exclusion of the six subjects born SGA in the preterm group (P = 0.011).
None of the blood pressure measurements correlated to eGFR.
Risk factors for reduced kidney volume after preterm birth
Neonatal serum creatinine (P = 0.35), serum C-reactive protein (S-CRP) (P = 0.72), dopamine administration (P = 0.51) or the presence of a patent ductus arteriosis (P = 0.33) did not correlate to renal volume. There was neonatal renal failure, defined as serum creatinine > 100 mmol/ml after 1 week postnatal age, in seven preterm children. The two with the highest serum creatinine values during their neonatal hospital stay had the smallest kidneys.
Discussion
Our study demonstrated that children born preterm had insignificantly smaller kidney volume at school age, after we had adjusted for BSA, gender and age. Children born full term but with growth restriction had a kidney size comparable to that of the children born preterm, but after adjustment for current body size, no difference was found in comparison with the control group. Renal function was similar in the three groups with different birth characteristics.
The strength of the study is that our three groups were well defined with regard to gestational age at birth and foetal growth restriction. The children born preterm are representative for extremely preterm births, with a mean gestational age of 27 weeks and a mean birth weight of 1,000 grams. Secondly, all examinations were performed by the same research nurse and all ultrasound determinations by the same physician.
The weaknesses of the study are the small sample size and that not all children could be examined for both renal function and kidney volume. The point estimate indicated a lower renal volume in children born preterm (7–13 ml). However, we were not able to show a significant difference in kidney volume when adjusting for BSA, gender and age. This could be due to limitations in power.
eGFR is only an estimate and underestimates the gold standard of inulin clearance. The value of k used to calculate eGFR is also dependent on muscle mass, which could have differed in the three groups. Another limitation is that the volumetric investigations of the kidney by ultrasound were performed 3 years after the renal function tests and blood pressure measurements were done [12.6 (0.3) years and 9.6 (0.3) years, respectively]. We cannot exclude the possibility that, at the time that we detected lower kidney volume, renal function could have deteriorated in school age children born preterm.
With regard to renal volume, our findings could not confirm those of Schmidt et al. [27], who found significantly smaller kidneys up to 18 months of age in 178 infants born with low birth weight, including preterm infants. Catch-up growth for kidney volume was significant except for the infants born preterm.
We did not find deterioration in renal function in the children born preterm as other studies have found. Keijzer-Veen et al. [28] clearly showed that preterm birth in combination with intrauterine growth restriction led to unfavourable renal function at 19 years of age in 422 subjects. Rodríguez-Soriano et al. [29] were not able either to demonstrate smaller kidney volume in 40 children born preterm at 6–12 years of age compared to estimated normal values; however, there was no volumetric estimation performed in the control group. On the other hand they found lower creatinine clearance in the children born preterm than in the control group.
Kidney volume is thought to be directly related to the number of nephrons, and, therefore, volumetric estimation of the kidney by ultrasound is an indirect measurement of the number of functional units in the kidney [30]. Autopsy studies have shown that there is a lack of compensation in nephron number and size in infants born with foetal growth restriction [4–6] as well as in children born preterm [31, 32]. The nephrons, however, undergo glomerular hypertrophy and mesangial proliferation. Final renal size should be influenced by the nephron size. Glomerular hypertrophy and, secondarily, relative increase in renal size could partly explain the lack of significant findings in the children with foetal growth restriction. Similar kidney function in the children born preterm suggests that the smaller kidneys due to fewer nephrons are still able to compensate by a greater utilisation of the remaining functional units of the kidney, but no signs of glomerular or tubular damage is present at this time.
We speculated that children born preterm have smaller kidneys due to the compromising postnatal period, with inadequate nutrition, a stressful environment, and medical complications. These factors, as well as the administration of nephrotoxic drugs such as indomethacin and aminoglycosides, could negatively affect normal nephrogenesis [33]. However, we could not confirm those speculations in our study.
In conclusion, children born preterm or small for gestational age at term showed no significant differences in glomerular filtration and renal volume from those born with normal birth weight at term. Although the power of this conclusion is limited by the small numbers, it points to alternative explanations for the increased risk of hypertension in people who were born too small and too early. Other mechanisms, such as differences in renal sodium handling or the sympathetic nervous system, may be equally or even more important than a reduction in nephron numbers and kidney size.
In the future, it could be of value to look at flow patterns of arterial and venous vessels to and from the kidney to be able to detect increased resistance as an indicator for structural changes in the kidneys’ micro-architecture. Continued follow-up of these children to a higher age is necessary, to detect eventual changes in kidney function as early as possible.
References
Barker DJ, Osmond C, Golding J, Kuth PD, Wadsworth MEJ (1989) Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298:564–567
Brenner BM, Garcia DL, Anderson S (1988) Glomeruli and blood pressure: Less of one, more the other? Am J Hypertens 1:335–347
Brenner BM, Chertow GM (1994) Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23:171–175
Hinchlife SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D (1992) The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 99:296–301
Manalich R, Reyes L, Herrera M, Melendi C, Fundora I (2000) Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int 58:770–773
Merlet-Benichou C, Gilbert T, Muffat-Joly M, Lelievre-Pegorier M, Leroy B (1994) Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol 8:175–180
Hughson MD, Douglas-Denton R, Bertram JF, How WE (2006) Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69:671–678
Silver LE, Decamps PJ, Korst LM, Platt LD, Castro LC (2003) Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am J Obstet Gynecol 188:1320–1325
Fan ZJ, Lackland DT, Lipsitz SR, Nicholas JS (2006) The association of low birth weight and chronic renal failure among Medicaid young adults with diabetes and/or hypertension. Public Health Rep 121:239–244
Hoy WE, Rees M, Kile E, Mathews JD, McCredie DA, Pugsley DJ, Wang Z (1998) Low birth weight and renal disease in Australian aborigines. Lancet 352:1826–1827
Vanpée M, Blennow M, Linné T, Herin P, Apeira A (1992) Renal function in very low birth weight infants: normal maturity reached during early childhood. J Pediatr 121:784–788
Johansson S, Norman M, Legnevall L, Dalmaz Y, Lagercrantz H, Vanpée M (2007) Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. J Intern Med 261:480–487
Edstedt Bonamy AK, Bendito A, Martin H, Andolf E, Sedin G, Norman M (2005) Preterm birth contributes to increased vascular resistance and higher blood pressure in adolescent girls. Pediatr Res 58:845–849
Kistner A, Celsi G, Vanpée M, Jacobson SH (2000) Renal function and blood pressure control in adult women born preterm or small for gestational age. Pediatr Nephrol 15:215–220
Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S (2005) Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation 112:3430–3436
Høgberg U (1997) Early dating by ultrasound and perinatal outcome. A cohort study. Acta Obstet Gynecol Scand 76:907–912
Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B (1996) Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 85:843–848
Morris SS, Victoria CG, Barros FC, Halpern R, Menezes, AMB, César JA, Horta BL, Tomasi E (1998) Length and ponderal index at birth: associations with mortality, hospitalizations, development and post-natal growth in Brazilian infants. Int J Epidemiol 27:242–247
Roberts JM, Pearson G, Cutler J, Lindheimer M (2003) Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 41:437–445
Schwartz GJ, Haycock GB, Edelman CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Park MK, Menard SM (1987) Accuracy of blood pressure measurement by the Dinamap monitor in infants and children. Pediatrics 79:907–914
Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte Wissermann H (1985) Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr Radiol 15:38–43
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height weight formula validated in infants, children and adults. J Pediatr 93:62–66
Lane PH, Belsha CW, Plummer J, Steinhardt GF, Lynch RE, Wood EG (1998) Relationship of renal size, body size, and blood pressure in children. Pediatr Nephrol 12:35–39
Srivastava T (2006) Nondiabetic consequences of obesity on kidney. Pediatr Nephrol 21:463–470
Saran AM, Hsu FC, Lohman KK, Carr JJ, Bowden DW, Wagenknecht LE, Freedman BI (2008) Kidney volume associations with subclinical renal and cardiovascular disease: the diabetes heart study. Am J Nephrol 28:366–371
Schmidt IM, Chellakooty M, Boisen KA, Damgaard IN, Mau KC, Olgaard K, Main KM (2005) Impaired kidney growth in low-birth-weight children: distinct effects of maturity and weight for gestational age. Kidney Int 68:731–740
Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frolich M, van der Heijden BJ (2005) Dutch POPS-19 Collaborative Study Group. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 9:2762–2768
Rodríguez-Soriano J, Aguirre M, Oliveros R, Vallo A (2005) Long-term renal follow-up of extremely low birth weight infants. Pediatr Nephrol 20:579–584
Nyengaard JR, Bendtsen TF (1992) Glomerular number and size in relation to age, kidney weight and body surface in normal man. Anat Rec 232:194–201
Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE (2004) Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7:17–25
Rodríguez MM, Gómez A, Abitbol C, Chandar J, Montané B, Zilleruelo G (2005) Comparative renal histomorphometry: a case study of oligonephropathy of prematurity. Pediatr Nephrol 20:945–949
Cuzzolin L, Fanos V, Pinna B, di Marzio M, Perin M, Tramontozzi P, Tonetto P, Cataldi L (2006) Postnatal renal function in the preterm newborns: a role of diseases, drugs and therapeutic interventions. Pediatr Nephrol 21:931–938
Acknowledgements
The study was funded by the May Flower Annual Campaign for Children’s Health, the Swedish Medical Association, Sällskapet Barnavård, Mjölkdroppen Foundation, Freemason Children’s Foundation in Stockholm, and the Swedish Heart and Lung Foundation. We sincerely thank the participating children and their families for making this study possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rakow, A., Johansson, S., Legnevall, L. et al. Renal volume and function in school-age children born preterm or small for gestational age. Pediatr Nephrol 23, 1309–1315 (2008). https://doi.org/10.1007/s00467-008-0824-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0824-z