Abstract
A reduced nephron complement at birth renders the kidney susceptible to renal disease in adulthood. Retinoic acid (RA; the active metabolite of vitamin A) is linked to nephrogenesis in vitro and in vivo. The aim of this study was to determine the effect of administration of retinoic acid in midgestation in rats on nephron endowment in offspring exposed to maternal protein restriction. Rats were fed either a normal-protein diet (NPD) or a low-protein diet (LPD) during pregnancy and lactation. Half of the dams in the LPD group were injected intraperitoneally with retinoic acid (20 mg/kg) during gestation at embryonic day 11.5. At 4 weeks of age, the offspring were anesthetized and perfusion-fixed, and nephron number estimated using unbiased stereological techniques. Body weight and kidney volume was significantly reduced in all LPD offspring. There was a significant 29% reduction in nephron number in the LPD group compared with the NPD offspring, whereas the number of nephrons in kidneys from the LPD + RA offspring was not significantly different compared with controls. In conclusion, administration of a single bolus dose of retinoic acid during midgestation restored nephron endowment to normal in offspring exposed to maternal protein restriction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrauterine growth restriction (IUGR) as evidenced by a low birth weight for gestational age is considered to be a predisposing factor for hypertension and renal disease [1–5]. In particular, low birth weight is thought to be a major contributing factor to the marked increase in the incidence of hypertension and renal disease in at-risk populations, such as the Australian Aborigines [5, 6].
In experimental studies, low birth weight due to IUGR results in significant reduction in the weight of many vital organs at birth, including the kidneys [7–10], and can ultimately lead to permanent abnormalities in renal structure [11, 12]. Of particular importance, IUGR often leads to reduced nephron endowment in the low-birth-weight infant [12, 13]. This may have serious clinical implications later in life, as nephrogenesis in humans is complete at birth [14], with no new nephrons formed after this time. Thus, whereas low-birth-weight infants may have postnatal catch-up growth, the nephrons of the kidney can only enlarge. In rodents, nephrogenesis is completed a few days after birth, with a full complement of nephrons developed by postnatal day 10 in rats [15]. Previously in our laboratory, we have shown that maternal protein restriction during pregnancy and lactation in rats leads to about a 30% reduction in nephron endowment in the offspring [16]. Other laboratories report similar findings in other similar models of IUGR [8, 17, 18].
Congenital reduction in nephron endowment at birth has been linked to the development of hypertension later in life [19]. Although recent experimental studies from our laboratory demonstrate that reduction in nephron endowment does not necessarily lead to hypertension in adulthood (due to adequate compensatory hypertrophy) [20, 21], we and others have recently shown that a congenital nephron deficit renders the kidney susceptible to subsequent post-natal renal insults [22, 23], and therefore it is imperative to maximize nephron endowment at birth. In this regard, there have been a number of studies linking vitamin A acting via the c-ret receptor, with the stimulation of nephrogenesis in the fetal kidney [24–29]. For instance, Lelievre-Pegorier et al. [25] showed that nephron endowment in the fetus is linearly correlated with circulating vitamin A levels, with a 50% reduction in circulating vitamin A in the fetus resulting in a 20% reduction in nephron endowment. Interestingly, a single injection of retinoic acid (RA) (the active metabolite of vitamin A) to a control group of pregnant rats at midgestation can lead to supernumerary nephron endowment in the kidneys of the offspring [25]. Hence, the aim of our study was to determine whether administration of RA during midgestation can restore nephron endowment in offspring exposed to maternal protein restriction to a level similar to that of control offspring.
Methods
Animals
Female and male Wistar Kyoto (WKY) breeder rats (at 12 and 10 weeks of age, respectively) were obtained from the Australian Resource Centre, Perth. The female rats were divided into three experimental groups: a control normal-protein-diet group (NPD), a low-protein-diet group (LPD) and a low-protein-diet-plus-retinoic-acid group (LPD + RA). The dietary regimes and the protocol for administering the RA are described in the next section. To generate the offspring used in this study, the dams were time-mated. To do this, the female rats were identified as being in estrous by vaginal smears. The male rat was then placed into the cage with the female and taken out the following morning. This was then considered as day 0.5 of gestation in the offspring, following confirmation of vaginal sperm in the dams. The dams were housed individually throughout pregnancy and maintained at an ambient temperature of 21°C. Food and water were administered ad libitum. At postnatal day 2, litters were reduced to eight pups. In reducing the litters, the pups that appeared to be runts of the litter were culled, and the remainder to be culled was randomly selected. At 4 weeks of age, the offspring (male and female) were assigned a number and randomly chosen for kidney analyses (n = 7 or 8 per group). It has previously been shown that there is no difference between the number of nephrons in male and female offspring [30, 31]. All animal experiments were approved by the Monash University, Biochemistry, Anatomy and Microbiology Animal Ethics Committee, and treatment and care of the animals conformed to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Administration of diets and retinoic acid
The female breeder rats were fed either an LPD (8.7% casein) or an NPD (20% casein) for 2 weeks prior to mating, during pregnancy, and for 4 weeks postpartum [21, 32]. The dams were maintained on the diets during lactation, as nephrogenesis is not complete in the rat until postnatal day 10 [15]. The semipurified diets (obtained from Glenn Forest Stockfeeders, Perth) differed only in their starch and casein content (see Table 1) [21, 32]. The amount of vitamin A in the diet did not differ between the LPD and NPD diets (Table 1).
All trans retinyl acetate (Sigma-Aldrich, Irvine, UK) (at a melting point of 58°C) was dissolved in corn oil to make a stock solution of 1 g/10 ml. Each female breeder rat in the LPD + RA group received an intraperitoneal injection of RA from the stock solution at a dose of 20 mg/kg on embryonic day 11.5 [25]. It must be kept in mind when designing strategies utilizing vitamin A supplementation during pregnancy that both low and high doses of vitamin A can be teratogenic [33, 34]. A pilot study was initially undertaken to confirm that the dose of RA administered to the pregnant rats raised serum vitamin A levels but was not teratogenic to the fetuses. In the pilot study, vitamin A levels in the pregnant dams were not affected by the administration of the LPD diet.
The dams from the NPD and LPD groups were injected intraperitoneally with corn oil vehicle, at a similar amount per body weight, at the same time point (embryonic day 11.5) in pregnancy. At 4 weeks of age (the time of weaning), the offspring were anesthetized and perfusion-fixed with 4% paraformaldehyde in a 0.1 molar phosphate buffer at a pressure of 100 mmHg. Prior to perfusion fixation, both heparin sodium (500 U in 0.1 ml IV) to reduce blood clotting and papaverine hydrochloride (1.2 mg in 0.1 ml) to dilate the vasculature were administered intraperitoneally [21]. On completion of fixation, the right kidneys were excised, decapsulated, assigned an experimental number, and stored in 10% buffered formalin.
Stereological estimation of kidney volume, nephron number, and glomerular size
Perfusion-fixed right kidneys (n = 7 or 8 per group) were sliced at 1 mm in the horizontal plane using a razor blade slicing device. Kidney slices were dehydrated through graded alcohols and embedded in glycolmethacrylate. The blocks were exhaustively sectioned at 20 μm. Every tenth (sampled section) and 11th (look-up section) were collected (with the first section between one and ten chosen at random), and stained with haematoxylin and eosin. The sampled sections (every tenth section) were projected onto a microfiche screen, an orthogonal grid was superimposed, and the volume of the kidney was estimated using the Cavalieri principle [35]. The number of glomeruli (and thereby nephrons) was determined using a physical disector/fractionator stereological approach. The numerical density, which is the number of nephrons per volume of kidney tissue, was also determined. These stereological methods are routine in our laboratory and have been described in detail previously [20, 36]. Also, using unbiased stereological techniques, mean glomerular and renal corpuscle volumes were determined as previously described [20, 36]
Statistical analysis
Data were analyzed using Graphpad Prism version 3.00 (Graphpad Software, USA). Prior to statistical analysis, all data were tested for normality and found to be normally distributed. Data were then analyzed using a one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test. Results are expressed as means ± standard error of the mean (SEM). Statistical significance was considered as a p value ≤0.05.
Results
Body weights of offspring
At termination of the experiment (4 weeks of age), there was a marked reduction in body weight in all offspring exposed to maternal protein restriction during pregnancy and lactation. Mean body weights averaged 61.3 ± 4.3 g in the NPD group, whereas mean body weights averaged 50.4 ± 1.5 g in the LPD group and 51.7 ± 0.9 g in the LPD + RA group. In accordance with the body-weight data at 4 weeks of age, kidney size was significantly lower in the LPD and LPD + RA groups compared with the NPD controls (Fig. 1). There was no statistical difference in kidney volumes between the LPD and LPD + RA groups (Fig. 1). When adjusted for body weight, there was no significant difference in the kidney volume-to-body weight ratio between groups (NPD: 4.14 ± 0.24 mm3/g; LPD: 4.37 ± 0.22 mm3/g; LPD+RA: 4.05 ± 0.14 mm3/g).
Total nephron number
At 4 weeks of age, there were significantly fewer (p < 0.001) glomeruli (and thereby nephrons) in the kidneys of the LPD offspring compared with the NPD and LPD + RA offspring (Fig. 2). The LPD offspring had approximately 29% fewer glomeruli than the NPD offspring and 22% fewer nephrons than the LPD + RA offspring. Interestingly, there was no difference in nephron number between the NPD group and the LPD + RA group. As a consequence, there was a significant increase (p < 0.001) in the numerical density of nephrons (number of nephrons per volume) in the kidneys of the LPD + RA offspring when compared with the NPD and LPD groups (Table 2).
Total nephron number per kidney at 4 weeks of age in offspring from the normal-protein diet (NPD) (■) (n = 7), low-protein diet (LPD) (●) (n = 8), and LPD + retinoic acid (RA) (▼) (n = 7) offspring. Data points represent individual animals, and the lines represent the mean number of glomeruli. **p < 0.001 compared with the NPD and LPD + RA groups
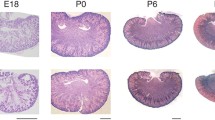
Microscopically, the kidney morphology looked similar between the groups; however, the increased density of glomeruli was apparent in the cortex of the kidneys from the LPD + RA group (Fig. 3).
Glomerular and renal corpuscle volume
Mean glomerular and renal corpuscle volumes are shown in Table 2. There was no statistical difference in mean glomerular volume or mean renal corpuscle volume in kidneys from the NPD, LPD, and LPD + RA offspring. However, it is to be noted that in the kidneys of the LPD group, where there were fewer nephrons compared with the other groups, there was a trend for glomerular volume to be enlarged, but this just failed to reach statistical significance (p = 0.0706).
Discussion
In this study, offspring exposed to maternal protein restriction during pregnancy and lactation had a significantly reduced body weight, kidney size, and nephron endowment at weaning. Importantly, administration of a bolus dose of RA at embryonic day 11.5 was able to restore nephron endowment to normal in offspring exposed to maternal protein restriction without affecting body weight or kidney size, such that the number of nephrons per volume of kidney tissue was increased in these animals above that seen in the kidneys of control NPD offspring.
There have been several studies both in vivo and in vitro demonstrating a stimulatory effect of RA on nephrogenesis [24–29]. The findings of the study reported here support this concept. Importantly, administration of RA during pregnancy led to an increase in the number of nephrons per mm3 of kidney tissue above that found in control kidneys. This is an interesting finding, as in a recent study in our laboratory, we found that the main determinant of nephron number during normal gestation is kidney size [37]. In that study in baboons, we found a remarkably strong correlation between kidney weight and nephron number (R 2 = 0.917, p = 0.0002) and kidney volume and nephron number (R 2 = 0.837, p = 0.001). Strong correlations between kidney size and nephron endowment have also been described in human kidneys [38]. Hence, the findings of the study reported here indicate that administration of RA during pregnancy, early in gestation, is able to stimulate nephrogenesis per volume of kidney tissue over and above control levels. In support of our results, similar findings have also been described in the offspring of Sprague-Dawley rats that were fed a normal-chow diet during pregnancy and administered a bolus dose of RA also at embryonic day 11.5 [25]. The kidneys of those offspring also had supernumerary nephron endowment and an increased density of nephrons within the kidneys when compared with control offspring. In that study by Lelievre-Pegorier et al. [25], administration of RA during pregnancy appeared to retard postnatal growth of the offspring, with body weights and kidney weights significantly reduced at postnatal day 14. However, in our study, postnatal growth did not appear to be affected by the administration of RA during fetal development. Body weights of the LPD + RA offspring were reduced compared with controls, but this appeared to be the result of maternal protein restriction, with body weights at 4 weeks of age significantly reduced in all offspring exposed to maternal protein restriction. There was no difference in body weights between the LPD and LPD + RA treatment groups.
The mechanisms by which RA stimulates nephrogenesis are not fully understood. In renal development, the formation of nephrons within the metanephros (the permanent kidney) commences when the ureteric bud invades the metanephric mesenchyme, with signals from the metanephric mesenchyme causing the ureteric bud to grow and undergo branching. Reciprocal signals from the ureteric bud then stimulate the metanephric mesenchyme to differentiate and form nephrons at the tips of the ureteric branches [39–41]. Studies from Merlet-Benichou’s laboratory suggest that RA mediates its effects on nephrogenesis by stimulating ureteric branching morphogenesis [24, 26]. These investigators suggest that the likely molecular candidate mediating these early nephrogenic effects is glial-cell-line-derived neurotrophic factor (GDNF), acting via its cell-surface receptor GDNF-α and subsequently activating the receptor tyrosine kinase c-ret [25]. Upregulation of c-ret is known to lead to increased branching morphogenesis of the ureteric bud and in turn enhance nephron formation [42, 43], and findings from Mendelsohn’s laboratory support the concept that vitamin A stimulates branching morphogenesis through c-ret [27, 29]. In embryonic kidney cultures, Moreau et al. demonstrated that RA leads to upregulation of c-ret expression, thus linking increased c-ret expression with the enhanced nephrogenesis [26]. Interestingly, this c-ret gene expression in cultured metanephroi is up-regulated by addition of RA in a dose-dependent manner, whereas GDNF gene expression remained unchanged [26].
Alternatively, administration of RA may mediate its effects on nephrogenesis via stimulation of the metanephric mesenchyme. In this regard, Welham et al. [44] reported an increase in apoptotic nuclei in the metanephric mesenchyme of the developing kidneys of rat offspring exposed to maternal protein restriction. Thus, their findings suggest that the reduced nephron formation in LPD offspring may be the consequence of reduced numbers of nephron precursor cells and/or loss of supportive interstitial precursors. Hence, if this is the case, an alternative explanation for our findings in this study is that administration of RA during pregnancy is able to prevent the increase in cellular apoptosis in the metanephric mesenchyme as a result of maternal protein restriction. Whether administration of RA acts to directly rectify mechanisms leading to impaired nephrogenesis as a result of maternal protein restriction or whether it acts via an independent mechanism could not be determined. The administration of RA to the NPD control dams would help to elucidate this. Clearly, further studies are required to elucidate the molecular mechanisms leading to the stimulation of nephrogenesis. The results from our study demonstrate that this is an ideal animal model in which to undertake such studies, and the addition of an NPD + RA group would be beneficial.
No doubt the timing of administration of RA during pregnancy is likely to influence the degree to which nephrogenesis is affected. In culture, it has been shown that the response to RA is significantly greater in embryonic kidneys derived from 13-day-old embryos compared with 14-day-old embryos [24]. In support of very early time points being critical for nephron endowment, studies by Wintour et al. [45] showed that dexamethasone administered to the sheep fetus at a mean age of 27 days’ gestation (term, 147 days) results in the sheep fetus having a low glomerular number (approximately 60% of that in control animals). Interestingly, at that early time point in the fetal lamb kidney, the ureteric bud has only just begun to invade the metanephric mesenchyme [46]. Taken together with the findings of our study, it is clearly evident that adverse or stimulatory triggers very early or just prior to the onset of nephrogenesis can have marked effects on nephron endowment at birth. Whether RA administration can also stimulate nephrogenesis at a time point late in gestation when nephrogenesis is well advanced or nearing completion is unknown and is important to determine in future studies.
In this study, we did not measure blood pressure or assess renal function in the offspring. We would expect, based on our previous findings [21], that blood pressure and renal function would not be different between offspring from the different groups at 5 weeks of age, even though nephron endowment is different. However, our recent findings [22] suggest that by restoring nephron endowment in LPD offspring that this would lead to a reduction in the relative risk of renal disease in adulthood. Further studies are required to determine whether this is the case.
In conclusion, this study demonstrated that RA is able to stimulate nephrogenesis in the IUGR fetus exposed to maternal protein restriction, thus restoring nephron endowment to levels similar to the non-growth-restricted fetus. However, as RA can be teratogenic at high doses, we do not advocate that RA be administered during pregnancy to women at risk of giving birth to a low birth weight infant. Instead, we propose that by using this experimental model, downstream molecules following RA stimulation of nephrogenesis can be identified, which may then provide subsequent therapeutic targets.
References
Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME (1989) Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298:564–567
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ (1989) Weight in infancy and death from ischaemic heart disease. Lancet 2:577–580
Lackland DT, Egan BM, Fan ZL, Syddall HE (2001) Low birth weight contributes to the excess prevalence of end-stage renal disease in African Americans. J Clin Hypertens 3:29–31
Luyckx VA, Brenner BM (2005) Low birth weight, nephron number and kidney disease. Kidney Int (Suppl) 97:S68–S77
Hoy WE, Rees M, Kile E, Mathews JD, Wang Z (1999) A new dimension to the Barker hypothesis: low birth weight and susceptibility to renal disease. Kidney Int 56:1072–1077
Thomas M (2005) Deprivation and dialysis: pathways to kidney failure in Australian Aborigines. Adv Chronic Kidney Dis 12:84–87
Goldstein RS, Hook JB, Bond JT (1979) The effects of maternal protein deprivation on renal development and function in neonatal rats. J Nutr 109:949–957
Merlet-Benichou C, Gilbert T, Muffat-Joly M, Lelievre-Pegorier M, Leroy B (1994) Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol 8:175–180
Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA (1996) Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci 91:607–615
Langley-Evans SC (1997) Maternal carbenoxolone treatment lowers birthweight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin Sci 93:423–429
Allen LH, Zeman FJ (1973) Influence of increased postnatal nutrient intake on kidney cellular development in progeny of protein-deficient rats. J Nutr 103:929–936
Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D (1992) The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 99:296–301
Merlet-Benichou C, Vilar J, Lelievre-Pegorier M, Moreau E, Gilbert T (1997) Fetal nephron mass: its control and deficit. Adv Nephrol Necker Hosp 26:19–45
Hinchliffe SA, Sargent PH, Howard CV, Chan YF, Van Velzen D (1991) Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64:777–784
Larsson L, Aperia A, Wilton P (1980) Effect of normal development on compensatory renal growth. Kidney Int 18:29–35
Zimanyi MA, Bertram JF, Black MJ (2000) Nephron number in the offspring of rats fed a low protein diet during pregnancy. Image Anal Stereol 19:219–222
Langley-Evans SC, Welham SJ, Jackson AA (1999) Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64:965–974
Gilbert JS, Lang AL, Grant AR, Nijland MJ (2005) Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol 565:137–147
Brenner BM, Garcia DL, Anderson S (1988) Glomeruli and blood pressure: less of one, more of the other? Am J Hypertens 1:335–347
Black MJ, Briscoe TA, Constantinou M, Kett MM, Bertram JF (2004) Is there an association between level of adult blood pressure and nephron number or renal filtration surface area? Kidney Int 65:582–588
Zimanyi MA, Bertram JF, Black MJ (2004) Does a nephron deficit in rats predispose to salt-sensitive hypertension? Kidney Blood Press Res 27:239–247
Zimanyi MA, Denton KM, Forbes JM, Thallas-Bonke V, Thomas MC, Poon F, Black MJ (2006) Developmental nephron deficit is associated with increased susceptibility to secondary renal injury due to advanced glycation end-products (AGEs). Diabetologia 49:801–810
Plank C, Ostreicher I, Hartner A, Marek I, Struwe FG, Amann K, Hilgers KF, Rascher W, Dotsch J (2006) Intrauterine growth retardation aggravates the course of acute mesangioproliferative glomerulonephritis in the rat. Kidney Int 70:1974–1988
Vilar J, Gilbert T, Moreau E, Merlet-Benichou C (1996) Metanephros organogenesis is highly stimulated by vitamin A derivatives in organ culture. Kidney Int 49:1478–1487
Lelievre-Pegorier M, Vilar J, Ferrier M-L, Moreau E, Freund N, Gilbert T, Merlet-Benichou C (1998) Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int 54:1455–1462
Moreau E, Vilar J, Lelievre-Pegorier M, Merlet-Benichou C, Gilbert T (1998) Regulation of c-ret expression by retinoic acid in rat metanephros: implication in nephron mass control. Am J Physiol 275:F938–F945
Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J (1999) Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126:1139–1148
Gilbert T, Merlet-Benichou C (2000) Retinoids and nephron mass control. Pediatr Nephrol 14:1137–1144
Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C (2001) Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27:74–78
Vehaskari VM, Aviles DH, Manning J (2001) Prenatal programming of adult hypertension in the rat. Kidney Int 59:238–245
Neugarten J, Kasiske B, Silbiger SR, Nyengaard JR (2002) Effects of sex on renal structure. Nephron 90:139–144
Brandt Corstius H, Zimanyi MA, Maka N, Herath T, Thomas W, van der Laarse A, Wreford NG, Black MJ (2005) Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res 57:796–800
Wilson JG, Roth CB, Warkany J (1953) An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat 92:189–217
Campbell JL Jr, Smith MA, Fisher JW, Warren DA (2004) Dose-response for retinoic acid-induced forelimb malformations and cleft palate: a comparison of computerised image analysis and visual inspection. Birth Defects Res B Dev Reprod Toxicol 71:289–295
Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorrensen FB, Vesterby A, West MJ (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394
Black MJ, Briscoe TA, Dunstan HJ, Bertram JF, Johnston CI (2001) Effect of angiotensin-converting enzyme inhibition on renal filtration surface area in hypertensive rats. Kidney Int 60:1837–1843
Gubhaju L, Black MJ (2005) The baboon as a good model for studies of human kidney development. Pediatr Res 58:505–509
Nyengaard JR, Bendtsen TF (1992) Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232:194–201
Saxen L (1987) Organogenesis of the kidney. Cambridge University Press, Cambridge, pp 1–34
Moritz KM, Wintour EM (1999) Functional development of the meso-and metanephros. Pediatr Nephrol 13:171–178
Kuure S, Vuolteenaho R, Vainio S (2000) Kidney morphogenesis: cellular and molecular regulation. Mech Dev 92:31–45
Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR (1996) Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc Natl Acad Sci USA 93:10657–10661
Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumae U, Meng X, Lindahl M, Pachnis V, Sariola H (1997) Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124:4077–4087
Welham SJ, Wade A, Woolf AS (2002) Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int 61:1231–1242
Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M (2003) Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549:929–935
Wintour EM, Alcorn D, Butkus A, Congiu M, Earnest L, Pompolo S, Potocnik SJ (1996) Ontogeny of hormonal and excretory function of the meso-and metanephros in the ovine fetus. Kidney Int 50:1624–1633
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makrakis, J., Zimanyi, M.A. & Black, M.J. Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr Nephrol 22, 1861–1867 (2007). https://doi.org/10.1007/s00467-007-0572-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-007-0572-5