Abstract
Cross-sectional studies have demonstrated that left ventricular hypertrophy (LVH) is common in children on maintenance dialysis. We report the echocardiogram results of 17 children from seven centers in the Midwest Pediatric Nephrology Consortium who have spent at least 2 years on maintenance dialysis and had three consecutive echocardiograms: at initiation of dialysis therapy and 1 and 2 years later. The results indicate that LVH is prevalent at the initiation of dialysis (82%) and remains both frequent (82%) and severe (59%) after 2 years of maintenance dialysis. Normalization of LV geometry was unlikely: the prevalence of concentric LVH increased and the prevalence of eccentric LVH did not change over time, indicating poor blood pressure and volume status control in these patients. We conclude that children on maintenance dialysis are at high risk for future cardiovascular disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A Special Report from the National Kidney Foundation Task Force on Cardiovascular Disease [1] identified left ventricular hypertrophy (LVH) to have high prevalence in adult patients with end-stage renal disease (ESRD) and to be associated with high cardiac morbidity and mortality. Similar to adults, children on maintenance dialysis frequently have LVH, which is often severe [2–4]. Most pediatric studies have been cross-sectional; therefore, it is currently unknown how LVH changes over time in children with ESRD. It is possible that persistent LVH might contribute to future cardiac disease, the leading cause of death in children and young adults who are exposed to long-standing dialysis [5, 6]. The relatively small number of patients who require long-term dialysis makes it difficult to assess LVH changes over time in a single pediatric center. We therefore performed a multicenter study in the Midwest Pediatric Nephrology Consortium (MWPNC) with the objective to assess changes in (1) LV Mass, (2) LV geometry, and (3) prevalence of LVH in children and adolescents on long-term dialysis.
Methods
This is a retrospective longitudinal analysis of echocardiographic data collected from pediatric patients receiving maintenance dialysis. Seven pediatric dialysis units from the MWPNC participated in the study. The Institutional Review Board of each participating center approved the study. Data were collected from November 2004 to November 2005. The inclusion criteria were: (1) children 18 years and younger on maintenance dialysis for at least 2 years; (2) no prior kidney transplantation; (3) baseline echocardiographic evaluation within 90 days after starting maintenance dialysis (echo 1); (4) two follow-up echocardiograms – at 1 and 2 years after the initiation of dialysis therapy. Seventeen subjects met the above criteria and were included in the analysis. The echocardiograms were performed at a mean of 2.2, 10.5, and 22.5 months following the initiation of dialysis. Patients with a history of disease that may affect cardiovascular structure or function (anatomic heart defects, congenital or familial cardiomyopathy) were excluded from the study.
Medical charts were reviewed for: gender, race, cause of ESRD, dialysis modality, medications (dose/day), including antihypertensives, steroids, and recombinant erythropoietin, as potential confounders for cardiac structure. Data collected at the time of echocardiography included: age, height, weight, and blood pressure. Laboratory results at the time of or most recent to echocardiography included: hematocrit, serum levels of intact parathyroid hormone (iPTH), calcium, phosphorus, bicarbonate, and albumin.
Echocardiographic data included: LV end-diastolic dimension, septal thickness, LV posterior wall thickness, and shortening fraction. Left ventricular mass (LVM) was calculated according to the American Society of Echocardiography criteria [7]. LVM index (g/m2.7) was used to evaluate LVH accounting for body size, as described elsewhere [8]. LVH was defined as LVM index greater than the 95th percentile for normal children and adolescents aged 5–18 years [9]. Because the 95th percentile values in young children are significantly higher than those in older children, the Cincinnati Children’s Hospital Medical Center reference values for LVM index were used for children younger than 5 years of age (four subjects in the study) [10]. Severe LVH was defined as LVM index greater than the 99th percentile in children less than 5 years of age and greater than 51 g/m2.7 for children older than 5 years [9, 10]. LVH and severe LVH status were reassessed at each time point according to age. Relative wall thickness (RWT) was calculated to assess the LV geometric pattern [11]. A value of 0.375 (95th percentile of control subjects) was used as the cutoff for normal [9]. Patients with LVH and elevated RWT were defined as having concentric LVH, and those with LVH and normal RWT had eccentric LVH. Concentric remodeling was defined as elevated RWT and normal LVM index.
Statistical analysis
The repeated measures ANOVA was used to compare serial values. Values are presented as the mean ± standard deviation (SD). The associations between variables were assessed by Spearman analysis using the SigmaStat ver. 3.1 statistical package (Systat Software, Point Richmond, Calif.). A p value ≤0.05 was considered to be statistically significant.
Results
At the time of initiation of dialysis, the average age was 8.5±6.0 years (range: 0.1–18 years). The majority of the participants were males (12; 70%) and white (eight; 47%); six (35%) were African-American and three (18%) were Asian. The primary kidney diagnosis included structural anomalies/familial diseases in nine (53%) patients, glomerulonephritis/focal segmental glomerulosclerosis (FSGS) and systemic immunological disease in six (35%) patients, and other disorders in two (12%) patients. Nine (53%) patients received hemodialysis and eight (47%) received peritoneal dialysis as the mode of renal replacement therapy. Eleven (65%) patients were treated with antihypertensive medications: eight patients were on a single medication, and three patients were taking two medications. Uncontrolled hypertension, defined as blood pressure above the age-, sex-, and height-specific 95th percentile [12], was present in nine (53%) patients during the baseline period.
The changes in clinical and echocardiographic data are presented in Table 1. There was no significant change in the mean values of blood pressure or any laboratory markers during the follow-up period. Fewer subjects had anemia, but the prevalence of hypertension did not change. There was no significant change in the mean LVM index from echo 1 to echo 2 or to echo 3. The prevalence of LVH at baseline was 82%. The overall prevalence of LVH did not change significantly from the time of the initiation of dialysis to the 2-year follow-up; the prevalence of severe LVH remained high during the follow-up period. Eccentric LVH remained the most common geometric pattern at all time points; the prevalence of concentric LVH increased but was not statistically significant.
At baseline, the mean LVM index tended to be higher in children with acquired kidney disease than in those with congenital kidney disease (62.7±13.1 g/m2.7 vs. 53.1±16.1 g/m2.7), in hypertensive patients than in normotensive patients (57.9±16.1 g/m2.7 vs. 48.2±13.2 g/m2.7) and in children on peritoneal dialysis than in those on hemodialysis (59.9±14.7 g/m2.7 vs. 52.3±16.3 g/m2.7), but the differences did not reach statistical significance (all p>0.05). Patients on peritoneal dialysis were significantly younger than patients on hemodialysis (3.5±3.7 vs. 11.5±5.3 years; p=0.007); there was no significant difference between groups for other clinical or laboratory parameters.
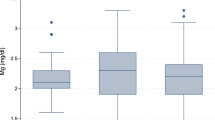
Despite no significant changes in the mean LVM index for the whole group during the study period, an examination of individual data revealed a large variability in the LVM index values (Fig. 1). Among three subjects with normal LVM index at baseline, two subsequently developed LVH; eight patients had a decrease in their LVM index; two subjects normalized LVM but six continued to have LVH. Correlation analysis showed that changes in the LVM index over time were significantly associated with changes in the systolic blood pressure (SBP) index: echo 1–echo 2, r=0.58 (p=0.02); echo 1–echo 3, r=0.68 (p=0.009). Analysis of the follow-up data according to dialysis modality showed no significant changes in the LVM index for either the hemodialysis (echo 2: 54.2±20.4 g/m2.7; echo 3: 53.2±19.3 g/m2.7, p=NS) or peritoneal dialysis (echo 2: 58.1±24.4 g/m2.7; echo 3: 56.6±22.7 g/m2.7, p=NS) group. There was no significant difference in the LVM index according to anemia status at any time point (data not shown).
Discussion
The majority of pediatric patients on maintenance dialysis receive kidney transplantation within a relatively short period of time after the initiation of dialysis therapy. However, there are a small number of dialyzed children who require long-term dialysis. These children are at risk for multiple complications and poor long-term prognosis. The survival of children with ESRD in the U.S. remains low, with an expected lifespan of 40–60 years less than that of an age- and- race- matched U.S. population [13]. The most notable cause of this poor survival is increased cardiac mortality due to the development of accelerated cardiovascular disease [5].
Even though children with ESRD usually do not present with symptomatic coronary artery disease or clinically evident congestive heart failure, many of them develop early cardiovascular abnormalities, including LVH, which is a known risk factor for cardiac failure and mortality. For this retrospective longitudinal and multicenter study, we selected a group of children who had spent at least 2 years on maintenance dialysis. We thought that these children might be at high risk for cardiac hypertrophy. The results of the study confirm that LVH remains very prevalent in children and adolescents after 2 years of maintenance dialysis. The results also indicate that normalization of LV geometry is uncommon: the prevalence of concentric LVH increased and the prevalence of eccentric LVH did not change, probably due to poor blood pressure and volume status control in these patients.
The major concern is that the severity of LVH did not decline in these children over 2 years of observation. Severe LVH, defined as an LVM index above 51 g/m2.7, is associated with a 4.1-fold increased risk of cardiovascular morbidity in adults [14]. In our subjects, LVH most likely reflected a continued compensatory process since the shortening fraction, which is used as a measure of systolic function, did not change during the follow up period. The long-term concern is that persistent and severe LVH in these dialyzed patients may eventually progress to a maladaptive stage of LVH with a risk for ultimate worsening of cardiac function and the development of fatal and nonfatal cardiovascular events [15]. Due to the limitations of the two-dimensional echocardiograms performed in these patients, we were unable to assess if these patients had diastolic dysfunction, which is frequently the initial abnormality of cardiac function seen with maladaptive LVH.
Previous studies in the adult dialysis population have shown that regression of LVH is possible with strict blood pressure control, but the majority of changes in the LVM index occur in the first year following the initiation of dialysis treatment [16]. In our study, despite a decrease in the LVM index in almost half of the children studied, no significant improvement in LVH status took place. Interestingly, the changes in the LVM index were significantly associated with the changes in blood pressure, suggesting that reduction of the LVM index indeed might be linked to an improvement in blood pressure. On other hand, the high prevalence of volume-dependent eccentric LVH suggests that fluid overload rather than hypertension per se is likely a cause of persistent LVH in our subjects. The higher LVM index in patients on peritoneal dialysis might also reflect a poorer volume control in these children, but we can not rule out that the younger age in peritoneal dialysis group is the reason for such results. The small sample size limited our ability to determine the specific risks for LVH.
We also recognize other limitations due to the retrospective and multicenter nature of the study. Specifically, we can not rule out that individual variability of echocardiographic data might in part be due to the lack of standardization among the centers and observer bias. The information on nephrectomy status, duration of chronic kidney disease prior to the initiation of dialysis, and fluid status were also not available. Despite these limitations, our report underscores the difficulty in gaining control of LVH in a selected group of children exposed to long-term dialysis. The results emphasize the need for careful monitoring and more aggressive management of volume overload and hypertension, especially within the initial months on maintenance dialysis when the opportunity to achieve blood pressure control and possibly reduce LVH in these patients is more optimal. Even more important, perhaps, is to intervene prior to dialysis since LVH is already very prevalent at the development of ESRD and seems to be difficult to improve once the patient is on dialysis.
References
Task force on cardiovascular disease (1998) Special report from the National Kidney Foundation. Am J Kidney Dis 32[Suppl 3]:1–121
Johnstone LM, Jones CL, Grigg LE, Wilkinson JL, Walker RG, Powell HR (1996) Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int 50:998–1006
Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Strife CF (2001) Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol 16:318–323
Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Meyer RA, Strife CF (2000) Severe left ventricular hypertrophy in pediatric dialysis: prevalence and predictors. Pediatr Nephrol 14:898–902
Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, Wolff ED, Davin JC, Heymans HS (2002) Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int 61:621–629
Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F (2002) Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106:100–105
Devereux RB, Reichec N (1977) Echocardiographic determination of left ventricular mass in man: anatomic validation of the method. Circulation 55:613–618
De Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitis O, Alderman MH (1992) Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20:1251–1260
Matteucci MC, Wuhl E, Picca S, Mastrostefano A, Rinelli G, Romano C, Rizzoni G, Mehls O, de Simone G, Schaefer F (2006) Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol 17:218–226
Khoury PR, Daniels SR, Gidding SS, Kimball TR (2004) Left ventricular mass index in children: what is the right index? J Am Soc Echo 17:555
de Simone G, Daniels SR, Kimball TR, Roman MJ, Romano C, Chinali M, Galdeirsi M, Devereux RB (2005) Evaluation of concentric left ventricular geometry in humans: Evidence for age-related systematic underestimation. Hypertension 45:64–68
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 2]:555–576
U.S. Renal Data System (2003) USRDS 2003 annual report. The National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
De Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH (1995) Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol 25:1056–1062
Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS (2004) Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int 65:1492–1498
Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE (1998) Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int 54:1720–1725
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitsnefes, M.M., Barletta, G.M., Dresner, I.G. et al. Severe cardiac hypertrophy and long-term dialysis: the Midwest Pediatric Nephrolgy Consortium study. Pediatr Nephrol 21, 1167–1170 (2006). https://doi.org/10.1007/s00467-006-0180-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0180-9