Abstract
Using data from systematic reviews and randomised controlled trials, the evidence for managing steroid sensitive nephrotic syndrome (SSNS) is reviewed. In the initial episode, increased duration (3–7 months) of prednisone compared with 2 months significantly reduced the risk for relapse at 12–24 months [relative risk (RR) 0.70; 95% confidence intervals (CI) 0.58–0.84] without increase in adverse effects. Six months of prednisone was significantly more effective than 3 months (RR 0.57; 95% CI 0.45–0.71). Higher prednisone doses given for the same duration reduced the risk of relapse (RR 0.59; 95% CI 0.42–0.84) suggesting that both dose and duration of prednisone therapy lead to prolonged remission. In relapsing SSNS prolonged prednisone treatment, daily prednisone during infections, oral or intravenous cyclophosphamide, chlorambucil, levamisole and cyclosporin significantly reduced the risk of relapse. Comparative effects of these options remain uncertain because of the absence of head-to-head trials, but existing trial evidence is strongest for cyclophosphamide and cyclosporin. Further adequately powered multinational trials are required to determine the optimum induction dose and duration of prednisone in the initial episode of SSNS and to determine the relative efficacies of immunosuppressive agents and the efficacy of newer agents, including mycophenolate and tacrolimus, in relapsing SSNS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic nephrotic syndrome is an uncommon condition in children with an incidence of 1–2 per 100,000 children aged below 16 years [1, 2]. The International Study of Kidney Disease in Childhood (ISKDC) determined the histopathological, clinical and laboratory characteristics of nephrotic syndrome in children [3]. Overall 87% of 521 children had idiopathic nephrotic syndrome with renal biopsy appearances of minimal change nephrotic syndrome (MCNS) in 76%. There is a close correlation between MCNS and response to steroids with 93% of children becoming free of proteinuria with 8 weeks of therapy [4]. Patients with other renal pathologies [5] may also achieve complete remission with steroids. Response to steroids is associated with a good long-term prognosis for renal function.
The long-term prognosis for most children with steroid-sensitive nephrotic syndrome (SSNS) is for resolution of their disease and maintenance of normal renal function. Approximately 80% of children with SSNS will relapse one or more times. Of those, 50% relapse frequently or become steroid dependent [6, 7]. The frequency of relapses decreases with time with 50–70% of children being relapse-free at 5 years and about 85% relapse-free at 10 years [6, 7]. Early relapse after initial treatment and short duration of remission increase the risk for subsequent relapse [8, 9]. Patients with frequent relapses during childhood are more likely to have disease persisting into adulthood [10].
Management of SSNS aims to induce and maintain complete remission without serious adverse effects of therapy. Steroid therapy is the primary therapy used to induce and maintain remission. Alkylating agents (cyclophosphamide, chlorambucil), cyclosporin and levamisole are commonly used to achieve prolonged periods of remission in children with frequently relapsing or steroid-dependent SSNS. The aim of this review is to evaluate the benefits and harms of currently used treatment regimens for the initial and subsequent episodes of SSNS.
What is the best evidence?
The best evidence for determining whether an intervention does more harm than good comes from randomised controlled trials (RCTs) or from systematic reviews of RCTs. Combining results from RCTs in systematic reviews provides an explicit method by which we can test whether the benefits and harms of interventions are constant across groups of patients or whether they vary. In addition, it may allow the efficacy of therapy to be demonstrated, when this is not evident from individual underpowered trials. In this review evidence from such studies will be considered primarily and evidence from lower grades of evidence only included where RCT evidence is not available. Specialised search strategies of major databases (including MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials) are used to identify RCTs in all languages for inclusion in a systematic review. Additional RCTs are identified by searching conference proceedings. The inclusion of all relevant RCTs is crucial to avoid bias in determining the efficacy of therapy since RCTs with negative results are less likely to be published. Where appropriate, results of trials are combined in meta-analyses, in which dichotomous data for individual trials may be expressed as relative risk (RR) or risk difference (RD) with 95% confidence intervals (CI) and a summary estimate RR or RD calculated. By convention, a summary estimate RR or RD with the upper limit of CI below one (the line of no effect) indicates that experimental therapy is more effective than control and/or reduces the incidence of an adverse effect. A summary estimate with its 95% CI crossing one indicate that no significant difference between therapies has been demonstrated.
Treatment of the first episode of SSNS
The ISKDC agreed on standard steroid regimens for the first episode of SSNS [11]. At presentation children received prednisone 60 mg/m2/day (maximum dose 80 mg) in divided doses for 4 weeks followed by 40 mg/m2/day (maximum 60 mg/day) in divided doses on 3 consecutive days out of 7 days for 4 weeks. Subsequent RCTs demonstrated that alternate day prednisone was more effective in maintaining remission than prednisone given on 3 consecutive days out of 7 days [12] and that there was no significant difference in the risk for relapse between single daily and divided doses of prednisone [13]. For reasons of ease of administration and compliance, a single daily dose is clearly the preferred option during daily therapy.
The ISDKC regimen is associated with a high relapse rate so that the efficacy of longer durations of steroids have been investigated in several RCTs, which have been combined in a systematic review [14, 15]. Treatment with prednisone for 3–7 months (administered daily for 4–8 weeks at 60 mg/m2/day and then on alternate days) compared with 2 months of therapy reduced the risk for relapse by 30% at 12–24 months (six trials; 422 patients; RR 0.70, 95% CI 0.58-0.84) (Fig. 1a) with a significant reduction in the number of children who relapsed frequently (RR 0.63, 95% CI 0.46-0.84). There were no significant differences in risks for adverse effects (Table 1). Other trials [14] have demonstrated that steroid therapy for 6 months significantly reduced the risk for relapse compared with 3 months (4 trials; 382 children; RR 0.57; 95% CI 0.45-0.71) (Fig. 1b). No additional benefit was demonstrated in one trial of treatment for 12 months compared with 5 months [16]. Duration of prednisone less than 2 months [17] was less effective than the standard regimen (one trial; 60 patients; RR 1.46, 95% CI 1.01–2.12). These data provide evidence that prolonged steroid therapy (5–7 months) should be given to reduce the risk for relapse following the first episode of SSNS.
Meta-analyses of the relative risk (95% confidence intervals) for relapse of nephrotic syndrome by 12–24 months in ( a) six trials comparing prolonged prednisone therapy (3–7 months) with 2-month therapy and in ( b) four trials comparing 6 with 3 months of prednisone therapy in children with the first episode of steroid-sensitive nephrotic syndrome. Results are shown ordered by trial weights. The test statistic Z indicates that increased duration of prednisone is significantly more effective in reducing the number of children who relapse compared with 2 months of prednisone (reproduced from Hodson et al. [14, 15] from the Cochrane Library with permission from John Wiley & Sons Ltd.)
Is dose or duration of steroid therapy important?
Increased duration of steroid therapy results in increased total dose of steroid therapy making the effects of duration and dose difficult to separate. Plotting the RR for relapse against the ratio of dose/duration suggested that duration was more important than dose [14]. However, two RCTs [18, 19] have compared different total doses of prednisone administered for the same duration (3 or 6 months). A meta-analysis of these studies showed that the risk of relapse was reduced by 40% with higher doses of steroids (RR 0.59; 95% CI 0.42–0.84) suggesting that both increased dose of steroids and prolonged duration are important in reducing the risk of relapse.
Applicability to individual patient care
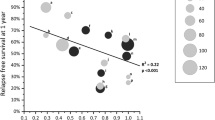
There is an inverse linear relationship between the risk for relapse and duration or total doses of induction therapy, suggesting an increase in benefit with treatment up to 7 months (Fig. 2). If 100% of children relapsed, the RR for relapse would fall by 11% per month for every 1 month increase in duration of therapy above 2 months (Table 2). Using the average relapse rate of 70% in children included in RCTs and treated for 2 months, the number of children relapsing by 12–24 months would fall by 7.7% for every increase by 1 month in the duration of therapy so that treatment for 6 months would reduce the risk of relapse by 31% (4×7.7%) to 39%.
Relationship between the risk of relapse (relative risk) by 12–24 months and the duration of prednisone given to children in the first episode of steroid-responsive nephrotic syndrome. Regression equation for duration of prednisone treatment: RR=1.26–0.11 duration; r 2=0.56 (reproduced from Hodson et al. [14, 15] from the Cochrane Library with permission from John Wiley & Sons Ltd.)
Alternative regimens in the first episode of SSNS
The Arbeitsgemeinschaft für Pädiatrische Nephrologie (APN) has demonstrated a reduction in RR for relapse with cyclosporin (150 mg/m2/day for 8 weeks) and prednisone compared with prednisone alone at 6 months (104 children; RR 0.33; 95% CI 0.13–0.83), but not at 1 or 2 years [14, 20]. The cumulative prednisone dose after 2 years was slightly but not significantly less in the cyclosporin-treated group, and no significant elevations in blood pressure or falls in glomerular filtration rate (GFR) were documented. The Chinese herb, Sairei-to, may be effective as a steroid sparing agent [14, 21]. Further trials are required before these regimens can be recommended for the initial episode of SSNS.
Treatment of frequently relapsing and steroid dependent SSNS
Steroid therapy
There are few data on steroid regimens for frequently relapsing and steroid-dependent SSNS. The ISKDC proposed that relapsing SSNS should be treated with daily prednisone (60 mg/m2/day) till the child had been in remission for 3 days followed by 4 weeks of prednisone given on 3 consecutive days out of 7. More recently, alternate day therapy has been preferred [12]. Important trial data are listed below.
Additional steroid therapy during intercurrent infections
Children commonly relapse when they have infections. Children with steroid-dependent SSNS had significantly fewer relapses during 2 years follow-up if they received daily rather than alternate-day prednisone during upper respiratory tract infections (36 children; mean difference −3.30; 95% CI −4.03 to −2.57) [14, 22].
Long duration regimens for relapsing SSNS
The risk of relapse at 1 year (76 children; RR 0.43; 95% CI 0.29–0.65) and at 2 years (64 children; RR 0.60; 95% CI 0.45–0.80) and the number of children with frequently relapsing or steroid-dependent SSNS (72 children; RR 0.43; 95% CI 0.20–0.95) were significantly reduced if children are treated for 7 months compared with the modified ISKDC regimen for relapsing patients [14, 23].
Deflazacort
Deflazacort [14, 15, 24] significantly reduced the number of children with steroid-dependent SSNS who relapsed during therapy (40 children; RR 0.44; 95% CI 0.25–0.78), without significant differences in side effects.
Pulse intravenous methylprednisolone
The number with relapse at 1 year was not significantly different (RR 1.06; 95% CI 0.75–1.52) when intravenous methylprednisolone (three doses of 20 mg/kg) followed by oral prednisone for 5 months was compared with oral prednisone for 6 months [14, 15, 25].
Non-steroid therapies
Alkylating agents
Both oral cyclophosphamide (2–3 mg/kg/day) and chlorambucil (0.2 mg/kg/day) administered for 8 weeks significantly reduce the risk of relapse in frequently relapsing SSNS (Table 3) [26, 27]. In a single comparison trial, cyclophosphamide and chlorambucil were equally effective [26, 27, 28]. The efficacy of cyclophosphamide administered for 12 weeks was not significantly different from 8 weeks [26, 27, 29] in an RCT though a study comparing treated patients with historical controls had suggested a benefit of treating for 12 weeks [30]. A recent trial [27, 31] has demonstrated that intravenous cyclophosphamide (500 mg/m2/dose for 6 monthly doses) was more effective than oral cyclophosphamide (2 mg/kg/day for 12 weeks) in reducing the risk for relapse at 6 months (RR 0.56; 95% CI 0.33–0.92), but not 2 years. Adverse effects did not differ between groups except that the children treated intravenously suffered fewer serious infections during treatment.
Adverse effects with alkylating agents are frequent and may be severe. In a systematic review Latta et al. [32] examined adverse effects from 38 reports, concerning 866 children receiving 906 courses of cyclophosphamide and 638 children receiving 671 courses of chlorambucil for frequently relapsing SSNS (Table 4). The authors concluded that chlorambucil in the recommended dosage was potentially more toxic than cyclophosphamide based on a higher risk of infections, malignancies and seizures. However, this conclusion was not based on comparative data from RCTs so differences in patient populations cannot be excluded. Gonadal toxicity with alkylating agents is more likely in boys than girls. In SSNS there is a dose-dependent relationship between the number of patients with sperm counts <106/ml and the cumulative dose of cyclophosphamide. The threshold cumulative dose for safe use remains uncertain because of individual reports of oligospermia in boys receiving less than 200 mg/kg. These data suggest that single courses of cyclophosphamide exceeding 12 weeks (2 mg/kg/day; cumulative dose 168 mg/kg), and second courses should be avoided if possible. There were few data on chlorambucil and the margin between effective treatment and a dose toxic to the male gonad may be smaller with chlorambucil than cyclophosphamide.
Cyclosporin
Single trials [26, 27, 33, 34] have demonstrated no significant difference in efficacy during treatment between cyclosporin and cyclophosphamide (55 children; RR 1.07; 95% CI 0.48–2.35) or cyclosporin and chlorambucil (40 children; RR 0.82; 95% CI 0.44–1.53). The majority of children treated with cyclosporin relapse when therapy is ceased. Adverse effects are significant with 4% of children developing hypertension, 9% reduced renal function, 28% gum hypertrophy and 34% hirsutism [26]. A retrospective study has shown that the administration of ketoconazole with cyclosporin as a cyclosporine-sparing agent reduced the number of children with renal impairment, increased the likelihood of withdrawing steroids and reduced the cost of therapy [35].
Levamisole
In three trials levamisole significantly reduced the risk for relapse in comparison with prednisone alone (three trials; 137 patients; RR 0.60; 95% CI 0.45–0.79) [27], but was ineffective in a fourth trial (48 patients; RR 1.18; 95% CI 0.96–1.46) [36]. Among the 3 trials [36, 37, 38], which enrolled frequently relapsing and/or steroid-dependent patients, the difference in efficacy may be related to the total dose administered (35 mg/m2/month [37, 38] versus 20 mg/m2/month [36]) and to the dose frequency (alternate day [37, 38] versus 2 consecutive days out of 7 days [36]). No trials have compared levamisole with other non-steroid agents. In a retrospective analysis of children with frequently relapsing or steroid-dependent SSNS, the mean number of relapses was reduced to an equivalent extent by levamisole treatment given for 6 months or more or cyclophosphamide given for 8–12 weeks [39]. Adverse effects of levamisole are uncommon, but include leucopenia, gastrointestinal effects and occasionally vasculitis [40, 41]. Unfortunately, the current manufacturers of levamisole have decided to cease its production.
Other agents
In RCTs, no significant reduction in the risk for relapse has been demonstrated with azathioprine [26], mizoribine [42], intravenous immunoglobulin [43] or sodium cromoglycate [44]. Mycophenolate mofetil [45, 46], tacrolimus [47], vincristine [48] and the ACE inhibitor, captopril [49], have been shown to reduce the risk of relapse in uncontrolled studies.
Conclusions
Information from meta-analyses of RCTs on the efficacy of interventions for steroid-sensitive nephrotic syndrome compared with the risks of some serious adverse effects is shown in Table 3. In children in their initial episode of SSNS, we know that the following therapies are likely to be more beneficial than harmful: 3 to 7 months of steroid therapy compared with 2 months and 6 months of steroid therapy compared with 3 months.
These data indicate that prolonged courses of steroid therapy should be administered in the first episode of SSNS to reduce the risk of early relapse and that this regimen, by reducing relapses, may result in a net reduction in steroid exposure compared with shorter courses.
In children with frequently relapsing and steroid-dependent SSNS, we know that the following therapies are likely to be beneficial: oral cyclophosphamide (2 mg/kg/day) for 8 weeks; oral chlorambucil (0.2 mg/kg/day) for 8 weeks; cyclosporin 6 mg/kg/day.
Though efficacy does not differ between cyclophosphamide and chlorambucil, serious adverse effects appear to be more common with chlorambucil, suggesting that cyclophosphamide should be used rather than chlorambucil (Tables 3, 4). Cyclosporin is as effective as alkylating agents during therapy, but has significant side effects (Table 3). These data suggest that either cyclophosphamide or cyclosporin may be used as the first non-steroid agent in children with relapsing SSNS with the choice based on treatment duration, side effect profile and availability.
In children with their first episode of SSNS, we need more information on the optimal duration and/or total dose of steroid therapy in terms of benefits and harms, the relative contributions of steroid duration and dose to efficacy and whether cyclosporin with 3 months of steroid therapy would be more effective and less toxic than 6 months of steroids. In children with frequently relapsing and steroid-dependent SSNS, we need more information on the efficacy of levamisole in frequently relapsing and steroid-dependent SSNS, the relative efficacies of alkylating agents, cyclosporin and levamisole in frequently relapsing and steroid-dependent SSNS, the relative efficacy of medications in steroid-dependent compared with frequently relapsing SSNS, the efficacy of mycophenolate compared with steroids alone or other non-steroid agents, the efficacy of tacrolimus compared with other non-steroid agents and the efficacy of angiotensin-converting enzyme inhibitors in comparison with steroids or non-steroid medications.
Paediatric nephrologists need further information on how to manage their patients from adequately powered randomised controlled trials. Since SSNS is a relatively rare condition in many countries, adequately powered international multicentre trials, such as the planned double-blind, placebo-controlled trial of levamisole [50], are required to answer these important questions.
References
Schlesinger ER, Sultz HA, Mosher WE, Feldman JG (1968) The nephrotic syndrome. Its incidence and implications for the community. Am J Dis Child 116:623–632
McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM (2001) Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 16:1040–1044
International Study of Kidney Disease in Children (1978) Nephrotic syndrome in children: Prediction of histology from clinical and laboratory characteristics at time of diagnosis. Kidney Int 13:159–165
International Study of Kidney Disease in Children (1981) The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. J Pediatr 98:561–564
International Study of Kidney Disease in Children (1981) Primary nephrotic syndrome in children: Clinical significance of histopathologic variants of minimal change and of diffuse mesangial proliferation. Kidney Int 20:765–771
Koskimies O, Vilska J, Rapola J, Hallman N (1982) Long-term outcome of primary nephrotic syndrome. Arch Dis Child 57:544–548
Tarshish P, Tobin JN, Bernstein J, Edelmann CMJ (1997) Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8:769–776
International Study of Kidney Disease in Children (1982) Early identification of frequent relapsers among children with minimal change nephrotic syndrome. J Pediatr 101:514–518
Takeda A, Takimoto H, Mizusawa Y, Simoda M (2001) Prediction of subsequent relapse in children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 16:888–893
Fakhouri F, Bocquet N, Taupin P, Presne C, Gagnadoux M-F, Landais P, Lesavre P, Chauveau D, Knebelmann B, Broyer M, Grunfeld J-P, Niaudet P (2003) Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis 41:550–557
Arneil GC (1971) The nephrotic syndrome. Pediatr Clin North Am 18:547–559
Anonymous (1979) Alternate-day versus intermittent prednisone in frequently relapsing nephrotic syndrome. A report of “Arbeitsgemeinschaft für Pädiatrische Nephrologie”. Lancet 1:401–403
Ekka BK, Bagga A, Srivastava RN (1997) Single- versus divided-dose prednisolone therapy for relapses of nephrotic syndrome. Pediatr Nephrol 11:597–599
Hodson EM, Knight JF, Willis NS, Craig JC (2005) Corticosteroid therapy for nephrotic syndrome in children. The Cochrane Database of Systematic Reviews Issue 1. Art. no.: CD001533. DOI: 10.1002/14651858.CD001533
Hodson EM, Knight JF, Willis NS, Craig JC (2000) Corticosteroid therapy in nephrotic syndrome: a meta-analysis of randomised controlled trials. Arch Dis Child 83:45–51
Arbeitsgemeinschaft für Pädiatrische Nephrologie (1988) Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet 1:380–383
Kleinknecht C, Broyer M, Parchoux B, Loirat C, Nivet H, Palcoux JB, Ami-Moussa R (1982) Comparison of short and long treatment at onset of steroid sensitive nephrosis (SSN). Preliminary results of a multicenter controlled trial for the French Society of Pediatric Nephrology. Int J Pediatr Nephrol 3:45
Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, Okuhara K, Suehiro F, Ohshima Y, Mayumi M for the West Japan Cooperative Study of Kidney Disease in Children (2000) Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome. Kidney Int 58:1247–1252
Pecoraro C, Caropreso MR, Malgieri G, Raddi G, Piscitelli A, Nuzzi F (2004) Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial. Pediatr Nephrol 19:C72
Arbeitsgemeinschaft für Pädiatrische Nephrologie (1999) Results of the nephrotic syndrome study VIII of the APN: New standard treatment versus new standard treatment plus 8 weeks cyclosporin A. Pediatr Nephrol 13:C26
Yoshikawa N, Ito H, Takehoshi Y, Honda M, Awazu M, Iijima K, Nakamura H, Seino Y, Takeda N, Hattori S, Matsuda I (1998) Standard versus long-term prednisolone with Sairei-to in childhood steroid-responsive nephrotic syndrome: a prospective controlled study. Jpn J Nephrol 40:587–590
Matoo TK, Mahmoud MA (2000) Increased maintenance corticosteroids during upper respiratory infection decrease the risk of relapse in nephrotic syndrome. Nephron 85:343–345
Jayantha UK (2004) Comparison of ISKDC regime with a 7-month steroid regime in the first attack of nephrotic syndrome. Pediatr Nephrol 19:C81
Broyer M, Terzi F, Lehnert A, Gagnadoux MF, Guest G, Niaudet P (1997) A controlled study of deflazacort in the treatment of idiopathic nephrotic syndrome. Pediatr Nephrol 11:418–422
Imbasciati E, Gusmano R, Edefonti A, Zucchelli P, Pozzi C, Grassi C, Della Volpe M, Performo F, Petrone P, Picca M, Claris Appiani A, Pasquali S, Ponticelli (1985) Controlled trial of methylprednisolone pulses and low dose oral prednisone for the minimal change nephrotic syndrome. BMJ 291:1305–1308
Durkan AM, Hodson EM, Willis NS, Craig JC (2001) Immunosuppressive agents in childhood nephrotic syndrome: a meta-analysis of randomised controlled trials. Kidney Int 59:1919–1927
Durkan A, Hodson EM, Willis NS, Craig JC (2001) Non-corticosteroid treatment for nephrotic syndrome in children. The Cochrane Database of Systematic Reviews Issue 4. Art. no.: CD002290. DOI: 10.1002/14651858.CD002290
Anonymous (1982) Effect of cytotoxic drugs in frequently relapsing nephrotic syndrome with and without steroid dependence. N Eng J Med 306:451–454
Ueda N, Kuno K, Ito S (1990) Eight and 12 week courses of cyclophosphamide in nephrotic syndrome. Arch Dis Child 65:1147–1150
Anonymous (1987) Cyclophosphamide treatment of steroid dependent nephrotic syndrome: comparison of eight week with 12 week course. Report of Arbeitsgemeinschaft für Pädiatrische Nephrologie. Arch Dis Child 62:1102–1106
Prasad N, Gulati S, Sharma RK, Singh U, Ahmed M (2004) Pulse cyclophosphamide therapy in steroid-dependent nephrotic syndrome. Pediatr Nephol 19:494–498
Latta K, von Schnakenburg C, Ehrich JHH (2001) A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 16:271–282
Ponticelli C, Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, Zachello G, Confalonieri R, Altieri P, Bettinelli A, Maschio G, Cinotti GA, Fuiano G, Schena FP, Castellani A, Della Casa-Alberighi O (1993) Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrol Dial Transplant 8:1326–1332
Niaudet P and the French Society of Paediatric Nephology (1992) Comparison of cyclosporin and chlorambucil in the treatment of steroid-dependent idiopathic nephrotic syndrome: A multicentre randomized controlled trial. Pediatr Nephrol 6:1–3
El-Husseini A, El-Basuony F, Mahmoud I, Donia A, Hassan N, Sayd-Ahmad N, Sobh M (2004) Co-administration of cyclosporine and ketoconazole in idiopathic childhood nephrosis. Pediatr Nephrol 19:976–981
Weiss R (1993) Randomized double-blind placebo controlled trial of levamisole for children with frequently relapsing/steroid dependent nephrotic syndrome. J Am Soc Nephrol 4:289
British Association for Paediatric Nephrology (1991) Levamisole for corticosteroid-dependent nephrotic syndrome in childhood. Lancet 337:1555–1557
Rashid HU, Ahmed S, Fatima N, Khanam A (1996) Levamisole in the treatment of steroid dependent or frequent relapsing nephrotic syndrome in children. Bangladesh Renal J 15:6–8
Alsaran K, Grisaru S, Stephens D, Arbus G (2001) Levamisole vs. cyclophosphamide for frequently-relapsing steroid-dependent nephrotic syndrome. Clin Nephrol 56:289–294
Palcoux JB, Niaudet P, Goumy P (1994) Side effects of levamisole in children with nephrosis. Pediatr Nephrol 8:263–264
Barbano G, Ginevri F, Ghiggeri GM, Gusmano R (1999) Disseminated autoimmune disease during levamisole treatment of nephrotic syndrome. Pediatr Nephrol 13:602–603
Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, Tanizawa T, Wada H, Maki S for the Pediatric Mizoribine Study Group in Japan (2000) A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney Int 58:317–324
Rowe PC, McLean RH, Ruley EJ, Salcedo JR, Baumgardner B, Zaugg B, Mellits ED, DeAngelis C (1990) Intravenous immunoglobulin in minimal change nephrotic syndrome: a crossover trial. Pediatr Nephrol 4:32–35
Trompeter RS, Thomson PD, Barratt TM, Soothill JF (1978) Controlled trial of disodium cromoglycate in prevention of relapse of steroid-responsive nephrotic syndrome of childhood. Arch Dis Child 53:430–432
Hogg RJ, Fitzgibbons L, Buick J, Bunke M, Ault B, Baqi N, Trachtman H, Swinford R on behalf of the Southwest Pediatric Nephrology Study Group (2004) Clinical trial of mycophenolate mofetil (MMF) for frequent relapsing nephrotic syndrome in children. Pediatr Nephrol 19:C66
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42:1114–1120
Sinha MD, Macleod R, Rigby E, Clark AG (2004) Treatment of severe steroid dependent nephrotic syndrome (SDNS) in paediatrics with tacrolimus therapy. Pediatr Nephrol 19:C72
Kausman JY, Yin L, Jones CL, Powell HR (2004) Vincristine in steroid-dependent nephrotic syndrome. Pediatr Nephrol 19:C99
Jayantha UK (2004) Captopril therapy in children with steroid dependent nephrotic syndrome and their long term follow up. Pediatr Nephrol 19:C98
Davin JC, Merkus MP (2005) Levamisole in steroid-sensitive nephrotic syndrome of childhood: the lost paradise? Pediatr Nephrol 20:10–14
Acknowledgements
This review was presented at the 13th Congress of the International Pediatric Nephrology Association, 29 August to 2 September 2004, Adelaide, Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00467-006-2156-1
Rights and permissions
About this article
Cite this article
Hodson, E.M., Craig, J.C. & Willis, N.S. Evidence-based management of steroid-sensitive nephrotic syndrome. Pediatr Nephrol 20, 1523–1530 (2005). https://doi.org/10.1007/s00467-005-1968-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-1968-8