Abstract.
Little is known about renal function in children with iron deficiency anemia. The purpose of this study was to investigate renal tubular function in these children. We compared renal tubular function in 20 children with iron deficiency anemia with 20 healthy age-matched controls. Blood and urine samples were obtained for hematological and biochemical analysis. Mean fractional excretion of sodium and mean urinary N-acetyl-β-D-glucosaminidase/creatinine were significantly higher in the children with iron deficiency anemia than in controls (P<0.05). Hemoglobin levels were negatively correlated with urinary N-acetyl-β-D-glucosaminidase/creatinine (r= -0.44, P=0.015), but were not correlated with fractional excretion of sodium (r= -0.29, P=0.13). There was no correlation between urinary N-acetyl-β-D-glucosaminidase/creatinine and fractional excretion of sodium (r=0.32, P=0.09). The results suggest that children with iron deficiency anemia have impaired renal tubular function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron deficiency anemia (IDA) is common in children who live in developing countries. This condition is known to negatively affect a child's growth and intellectual development [1]. Iron deficiency has deleterious effects on cell respiration, mitochondrial oxidative properties, and the electron transport chain [2]. The possible impact of IDA on renal function has not been investigated widely. Since renal ischemia is characterized predominantly by tubular damage, we hypothesized that children with IDA might develop tubular dysfunction due to chronic renal hypoxia. Hypothetically, disturbed mitochondrial energy metabolism in IDA might also cause renal tubular cell dysfunction. Therefore we designed a study to assess renal function in children with IDA.
Patients and methods
The study was approved by the ethics committee of the Faculty of Medicine at Başkent University. Twenty pediatric patients with IDA (8 males and 12 females, mean age 25±9 months, age range 6–70 months) were enrolled, and 20 healthy children (6 males and 14 females, mean age 30±11 months, age range 7–72 months) served as controls. A blood sample was collected from each subject to determine hemoglobin (Hb) level, hematocrit (Hct), mean corpuscular volume (MCV), and red blood cell distribution width (RDW). Healthy children with normal Hb, Hct, MCV, and RDW served as controls. If Hb, Hct, MCV, and RDW suggested IDA (Hb<10.5 g/dl, Hct<32%, MCV<72 fl, RDW>15%), a second blood sample was drawn to determine the levels of serum iron, iron-binding capacity, and ferritin. Transferrin saturation was also calculated from the formula of serum iron level/iron-binding capacity level x 100. Criteria for iron deficiency anemia were: serum iron level<50 µg/dl, iron binding capacity >352 µg/dl, ferritin level <12 ng/ml, and transferrin saturation <15%. Serum sodium and creatinine were also determined in all subjects, including controls.
Fresh second-morning urine samples were obtained from subjects in both groups. Immediately after collection, an aliquot from each sample was frozen for enzyme analysis, and the remaining urine was cultured, microscopically examined, and tested for osmolality and levels of protein, glucose, microalbumin, creatinine, sodium, and calcium. Patients with urinary infection or malnutrition were excluded. Urinary N-acetyl-β-D -glucosaminidase (NAG) was measured in the frozen sample using the colorimetric method.
Statistics
Statistical analysis was performed using a computer-based program (SPSS for Windows, version 10). Data were expressed as mean±SD. Findings in the patient and control groups were compared using the Mann-Whitney U test. Correlations were assessed with Spearman's correlation coefficient test. A two-tailed P value <0.05 was accepted as statistically significant.
Results
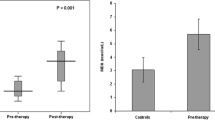
In the IDA patient group, the mean Hb, Hct, MCV, and RDW values were 9.6±0.8 g/dl (range 7.8–10.5 g/dl), 28.8±2.6% (range 21–31.3%), 65±6.4 fl, and 18.1±1.6%, respectively. The corresponding findings for the control group were 12.1±0.5 g/dl (range 11.4–13.2 g/dl), 35.8±1.6% (range 34–39.3%), 77.5±3 fl, and 13.8±0.7%, respectively. For all these parameters, the patient and control results were significantly different (P<0.001 for all). In the IDA patient group, the mean levels of iron, iron-binding capacity, and ferritin were 36±9 µg/dl (range 17–49 µg/dl), 366±67 µg/dl (range 388–450 µg/dl), and 4.7±3 ng/ml (range 1.2–12 ng/ml). There were no differences between the patient and control means for serum sodium (138.4±3 mEq/l versus 137.6±3.9 mEq/l, respectively), serum creatinine (0.49±0.1 mg/dl versus 0.48±0.1 mg/dl), or urine osmolality (588±211 mosmol/kg versus 590±256 mosmol/kg). The results of the IDA group for urinary microalbumin and calcium/creatinine ratio (Ca:Cr) were 0.83±0.75 mg/dl and 0.24±0.1, respectively, and the corresponding control findings were 1.33±1 mg/dl and 0.19±0.2 (P>0.05 for both comparisons). The fractional excretion of sodium (FENa) was significantly higher in the IDA group than in the controls [1.05±0.58% (range 0.1–2.1%) versus 0.6±0.2% (range 0.2–0.9%), respectively. P<0.05]. Also, urinary NAG/creatinine [UNAG/Cr (U/mg)] was significantly higher in patients with IDA than in controls [24.8±20 (range 5.7–69) versus 16.10±21.9 (range 3–89), respectively, P<0.05] (Table 1). Hemoglobin levels were negatively correlated with UNAG/Cr (r= -0.44, P=0.015), but were not correlated with FENa (r= -0.29, P=0.13). There was no correlation between UNAG/Cr and FENa (r=0.32, P=0.09).
Discussion
Researchers have investigated the deleterious effects of IDA on the cardiovascular and nervous systems, but the impact of this condition on renal function has not been examined in depth. In this study, although there were no differences between the IDA and control groups regarding serum sodium and creatinine, urine osmolality, urine microalbumin level, and Ca/Cr, increased FENa and UNAG/Cr were observed in the children with IDA.
The proximal tubule reabsorbs three times the sodium that the distal tubule reabsorbs [ 3], and this is why increased FENa reflects mainly proximal tubular damage. Sodium reabsorption is the primary determinant of renal oxygen consumption [4], thus the increased sodium excretion in our anemic patients may have been due to low cortical tissue oxygenation. Although FENa increased in the anemic patients, we could not find a negative correlation between Hb levels and FENa. We explained this finding by our patients' mild-to-moderate anemia status. We hypothesized that if the patients in the anemic group had had more severe IDA, a negative correlation would have been found between Hb levels and FENa.
Research has shown that NAG, a lysosomal enzyme found in proximal tubule cells, is a reliable indicator of proximal tubular damage [5]. Urinary excretion of NAG is increased in a variety of diseases and disorders that involve impaired proximal renal tubule function. In our study, Hb levels were negatively correlated with UNAG/Cr and the patients with IDA had a higher mean UNAG/Cr than the controls, indicating proximal tubule damage in the anemic group.
The literature contains little information about the morphological effects of anemia on the kidney. Kaissling et al. [6] demonstrated that the most striking anemia-related morphological change was damage to the proximal tubule. In tissues from anemic rats, the authors observed focal proximal tubular necrosis in the renal cortex but no structural signs of hypoxia in the medulla. In line with this, our clinical findings in children with IDA indicated proximal renal tubular dysfunction.
We hypothesized that the renal tubular dysfunction in our patients with IDA may have developed secondary to chronic hypoxia in the kidney. There is abundant blood flow in the renal cortex [7]; thus, one would not expect the kidney to become hypoxic as long as blood flow is normal. Studies have shown that the kidney consumes only 8–10% of the amount of oxygen delivered; thus, renal oxygen supply far exceeds demand [3]. However, it is well known that cortical tissue is very sensitive to hypoxic conditions, and that peritubular fibroblasts in the renal cortex produce erythropoietin during hypoxia [8]. Some researchers have questioned whether the kidney actually does have a rich oxygen supply, because low oxygen pressure has been documented in the renal cortices of anemic dogs [9] and non-anemic rats [10]. This finding in rat renal cortex may be explained by an oxygen shunt between the intrarenal arteries and veins, which results in lower cortical oxygen pressure than venous oxygen partial pressure [10]. This is an example of one situation where, even under normal blood flow conditions, anemia could lead to kidney hypoxia.
Mehta et al. [11] assessed renal function in 20 adults with IDA. They found that renal function, as measured by 3-h creatinine clearance was impaired in IDA, and that it improved after 3 days of intravenous iron therapy. Since no significant Hb rise was observed with iron administration, the authors speculated that iron effects at the tissue level might have caused the decreased creatinine clearance. Iron is vital to the function of iron-dependent enzymes including mitochondrial cytochromes, which participate in ATP generation. It has been reported that iron deficiency led to an oxidant-induced damage to mitochondria in rat liver [12]. As far as we know, the effect of iron deficiency on renal tissue has not been studied. Renal iron deficiency itself might disturb mitochondrial energy metabolism and affect renal tubular function.
In conclusion, the results of this preliminary investigation suggest that patients with IDA do exhibit proximal tubular damage and dysfunction. The cause of the dysfunction is not known. Reduced nutrient and oxygen delivery to cortical tubular cells, altered cell function due to anemic hypoxia, or iron deficiency in renal tissue may be key factors. Further studies are needed to determine the cause(s) of renal tubular dysfunction in IDA.
References
Hurtado EK, Claussen AH, Scott KG (1999) Early childhood anemia and mild or moderate mental retardation. Am J Clin Nutr 69:115–119
Bridges KR (1993) Iron metabolism and sideroblastic anemia. In: Nathan DG, Oski F (eds) Hematology of infancy and childhood, vol 1. Saunders, Philadelphia, pp 391–412
Gullans SR, Hebert SC (1996) Metabolic basis of ion transport. In: Brenner BM (ed) The kidney, 5th edn. Saunders, Philadelphia, pp 211–246
Piroso E, Erslev AJ, Flaharty KK, Caro J (1991) Erythropoietin life span in rats with hypoplastic and hyperplastic bone marrows. Am J Hematol 36:105–110
Kunin CM, Chesney RW, Craig WA, England AC, DeAngelis C (1978) Enzymuria as a marker of renal injury and disease: studies of N-acetyl-b-glucosaminidase in the general population and in patients with renal disease. Pediatrics 62:751–759
Kaissling B, Spiess S, Rinne B, Le Hir M (1993) Effects of anemia on morphology of rat renal cortex. Am J Physiol 264:F608-F617
Dworkin LD, Brenner BM (1996) The renal circulations. In: Brenner BM (ed) The kidney, 5th edn. Saunders, Philadelphia, pp 247–285
Lacombe C, DaSilva JL, Bruneval P, Fournier JG, Wendling F, Casadevall N, Camilleri JP, Bariety J, Varet B, Tambourin P (1988) Peritubular cells are the site of erythropoietin synthesis in the murine hypoxic kidney. J Clin Invest 81:620–623
Aperia AC, Liebow AA, Roberts LE (1968) Renal adaptation to anemia. Circ Res 22:489–500
Schurek HJ, Jost U, Baumgartl H, Bertram H, Heckmann U (1990) Evidence for a preglomerular oxygen diffusion shunt in the rat renal cortex. Am J Physiol 259:F910-F915
Mehta BC, Patel KB, Mehta JB (1989) Effect of iron deficiency on renal function. J Assoc Physicians India 37:685–686
Walter PB, Knudson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, Ames BN. (2002) Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc Natl Acad Sci U S A 99:2264–2269
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özçay, F., Derbent, M., Aldemir, D. et al. Effect of iron deficiency anemia on renal tubular function in childhood. Pediatr Nephrol 18, 254–256 (2003). https://doi.org/10.1007/s00467-002-1054-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-002-1054-4