Abstract
Objectives
To assess iodine and iron nutritional status among Nepalese school children.
Methods
A cross-sectional, community based study was conducted in the two districts, Ilam (hilly region) and Udayapur (plain region) of eastern Nepal. A total of 759 school children aged 6–13 y from different schools within the study areas were randomly enrolled. A total of 759 urine samples and 316 blood samples were collected. Blood hemoglobin level, serum iron, total iron binding capacity and urinary iodine concentration was measured. Percentage of transferrin saturation was calculated using serum iron and total iron binding capacity values.
Results
The mean level of hemoglobin, serum iron, total iron binding capacity, transferrin saturation and median urinary iodine excretion were 12.29 ± 1.85 g/dl, 70.45 ± 34.46 μg/dl, 386.48 ± 62.48 μg/dl, 19.94 ± 12.07 % and 274.67 μg/L respectively. Anemia, iron deficiency and iodine deficiency (urinary iodine excretion <100 μg/L) were present in 34.5 %, 43.4 % and 12.6 % children respectively. Insufficient urinary iodine excretion (urinary iodine excretion <100 μg/L) was common in anemic and iron deficient children.
Conclusions
Iron deficiency and anemia are common in Nepalese children, whereas, iodine nutrition is more than adequate. Low urinary iodine excretion was common in iron deficiency and anemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple micronutrient deficiencies continue to be the major nutritional problem in the developing countries [1]. Iodine and iron are two essential micronutrients whose deficiencies have adverse effects on growth and development, especially in pregnant women and children. Iron deficiency is the main cause of anemia and is a frequent cause of psychomotor disorders, poor coordination and decreased physical activity, whereas iodine deficiency has remained as the most common cause of preventable brain damage in children worldwide [2, 3].
Nepal demographic and health survey in 2006 revealed that iron deficiency manifested mostly as iron deficiency anemia (IDA), has a prevalence of nearly 48 % in 6–59 mo-old children of Nepal [4]. Studies by Baral et al. in 2009 and Khatiwada et al. in 2012, reported anemia in 65.6 % adolescents and 37.9 % children respectively, and a large number of children are presumed to suffer from iron deficiency [5]. Iodine nutrition has been improving in Nepal during the past decades.The proportion of school aged children with urinary iodine excretion (UIE) <100 μg/L decreased from 35 % in 1998 to 19.4 % in 2007 and iodized salt supplementation has been shown to improve iodine nutrition; yet sustainable elimination of iodine deficiency remains to be achieved [6].
Iodine and iron deficiencies are also often found coexisting. Extensive data from animal studies have shown that iron deficiency, with or without anemia, impairs thyroid metabolism [7]. Iron status has not been described previously for Nepalese children. Thus, considering the limited data on iron status, high vulnerability of children towards micronutrient deficiencies, and the reported coexistence of iodine and iron deficiencies in many communities, the present study was conducted. School children were enrolled randomly from the two districts of eastern Nepal, and urine and blood samples were analyzed to assess iodine and iron status.
Material and Methods
A community based, cross-sectional study was conducted among children aged 6–13 y in the year 2013 in the two districts (Ilam and Udayapur) of eastern Nepal to assess anemia, iron status and iodine status by measuring children’s blood hemoglobin level, serum iron, serum total iron binding capacity and urinary iodine. The two districts were selected randomly, as a representative of plain (Udayapur) and hilly (Ilam) districts, from total of 16 districts in eastern Nepal. A total of 759 children aged 6–13 y (325 and 434 from Ilam and Udayapur respectively), whose parents gave consent were randomly selected from different schools (5 schools in each district) within the study districts. The authors first selected schools (government and private) randomly from among the list of schools in each district, and then selected children randomly from each school. Children who were apparently healthy and did not have any serious illness were enrolled. The exclusion criteria was children taking any form of micronutrient supplements. The ethical clearance for this study was provided by the Institute Review Board of B P Koirala Institute of Health Sciences (BPKIHS) in 2013. The study sample size was calculated on the basis of prevalence of iodine deficiency (approximate 15 %) and iron deficiency (approximate 35 %) among Nepalese children.
Anthopometric variables (weight, height) were noted and casual urine samples (5–10 ml) were collected from all 759 children who participated in the study. However, venous blood samples (3 ml) were collected only from 316 school children, a subpopulation group made by random selection of subjects (166 and 150 from Udayapur and Ilam, respectively) from total study population. Urine samples were collected in a sterile tight screw capped plastic vials and venous blood was collected in the sodium EDTA tubes (1 ml) and plain vials (2 ml). The samples were transported to the biochemistry laboratory of BPKIHS maintaining cold chain. Hemoglobin was estimated within 24 h of sample collection and urinary iodine, serum iron and total iron binding capacity were estimated within a week of sample collection. Cyanmethemoglobin method was used to estimate blood hemoglobin (Hb) level [8]. Urinary iodine excretion (UIE) was measured by using ammonium persulphate digestion method (APDM) which is based on the principle of Sandell Kolthoff reaction [9]. Serum iron and total iron binding capacity (TIBC) were measured by colorometric methods using commercial kits (Roche Diagnostics) and transferrin saturation was calculated using serum iron and TIBC value [10]. Children were classified as having insufficient UIE (iodine deficiency) or sufficient UIE on the basis of UIE cutoff of 100 μg/L [11], into anemic or non-anemic groups on the basis of hemoglobin cutoff value for different age groups, and into iron deficient or iron sufficient groups on basis of transferrin saturation value. The hemoglobin levels less than 11.0 g/dl, 11.5 g/dl and 12.0 g/dl were considered as anemia for age groups 6–59 mo, 5–11 y and >12 y respectively. Similarly, transferrin saturation value less than 16 % was considered as iron deficiency condition [12]. Data from the study were entered in MS Excel 2007 and Statistical Package for Social Sciences (SPSS) version 11 (SPSS Inc., Chicago USA) was used for statistical analysis. The data was expressed as mean ± SD except for UIE. Independent t test and Mann Whitney test was applied for continuous variables at 95 % confidence level. For categorical variables Chi square test was applied at 95 % confidence level. Pearsons correlation analysis (for normally distributed variables) and Spearmans rank correlation analysis (non normally distributed variables) were done for calculating correlation among the variables.
Results
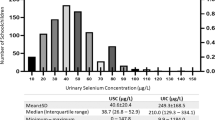
The study population comprised 759 children from rural and urban areas of the two districts of Nepal (Ilam and Udayapur). Children population was heterogeneous, comprising children of different ethnicity (major castes included Brahmin, Chhetri, Tharu, Rai/Limbu, Newar, other Terai origin and other castes), and with different economic status. The mean age, weight and height of study population were 9.2 ± 1.9 y, 24.99 ± 6.32 kg and 127.19 ± 11.7 cm respectively. Characteristics of study population according to gender and districts are shown in Table 1. The median UIE in total population was 274.67 μg/L. The mean level of Hb, serum iron, TIBC and transferrin saturation were 12.29 ± 1.85 g/dl, 70.45 ± 34.46 μg/dl, 386.48 ± 62.48 μg/dl and 19.94 ± 12.07 % respectively in the study sub-population. Children having insufficient UIE (UIE < 100 μg/L, which is considered as iodine deficiency on basis of WHO criteria) in the total population were 12.6 % (n = 96). Anemia and iron deficiency in the study subpopulation were found in 34.5 % (n = 109) and 43.4 % (n = 137) children respectively. No significant differences in iodine status, anemia and iron status were observed between males and females (p = 0.51, 0.42 and 0.89 respectively) and in between the two districts (p = 0.98, 0.51 and 0.068 respectively).
Children with UIE <100 μg/L had significantly lower Hb (11.47 ± 1.95 g/dl vs. 12.47 ± 1.78 g/dl; p = 0.001) and transferrin saturation (13.44 ± 8.97 % vs. 21.34 ± 12.21 %; p < 0.001) than those with UIE >100 μg/L. Anemic children had significantly lower serum iron (39.16 ± 18.9 μg/dl vs. 86.93 ± 28.94 μg/dl; p < 0.001), higher TIBC (436.83 ± 48.9 μg/dl vs. 359.97 ± 51.66 μg/dl; p < 0.001) and lower transferrin saturation (9.56 ± 6.44 % vs. 25.4 ± 10.68 %; p < 0.001) than non-anemic children. Anemia was significantly common in iron deficient (72.26 %, n = 99) than iron sufficient children (5.6 %, n = 10) (p < 0.001). Iron deficiency, with and without anemia was present in 31.3 % (n = 99) and 12.0 % (n = 38) children respectively. The relative risk for iron deficiency in anemic children was 12.93 (95 % CI: 7.02–23.83; p < 0.001) and the relative risk for anemia in iron deficient children was 4.94 (95 % CI: 3.68–6.63; p < 0.001). Hemoglobin (Hb) had significant positive correlation with serum iron (r = 0.576, p < 0.001) and transferrin saturation (r = 0.565, p < 0.001) and significant negative correlation with TIBC (r = −0.483, p < 0.001).
Median UIE and iodine status in anemic and iron deficient children is shown in Table 2. In the subpopulation of 316 children, 17.72 % (n = 56) had UIE <100 μg/L. Iodine deficiency in anemic and iron deficient children was 30.27 % (n = 33) and 26.27 % (n = 36) respectively. Median UIE was significantly different among iron deficient and iron sufficient children (p = 0.007) and among anemic and non-anemic children (p < 0.001). UIE had significant positive correlation with Hb (r = 0.313, p < 0.001), serum iron (r = 0.136, p = 0.016) and transferrin saturation (r = 0.126, p = 0.025), and weak negative correlation with TIBC (r = −0.107, p = 0.057).
Discussion
Micronutrient deficiencies especially affect the population in the growing phase. These deficiencies often co-exist along with other macronutrient and vitamin deficiencies [13]. The authors studied two important micronutrients, iodine and iron and their coexisting deficiencies in Nepalese children. The study revealed that 34.5 % and 43.4 % children in eastern Nepal have anemia and iron deficiency respectively. Non-iron deficient and non-anemic, iron deficient but non-anemic, and iron deficient and anemic children were 53.48 %, 12.02 % and 31.32 % respectively in the study population. Though previous data are unavailable for iron deficiency in Nepalese children, yet iron deficiency is considered to be highly prevalent in Nepal, especially among young children, pregnant women and teenage girls [14]. In a previous study done by authors’ team in eastern Nepal in 2012, anemia was observed in 37.9 % children, which is slightly higher than the current finding [5]. In one study in children aged 4–17 mo of South Central Nepal, Siegel et al. reported that 58 % children have anemia and 43 % have iron deficiency anemia [15].
The median UIE in the present study was 274.67 μg/L, which indicates that children have more than adequate iodine nutrition. Universal salt iodization program has been much successful in decreasing iodine deficiency throughout the country. Latest report shows that 85 % of households in eastern Nepal consume adequately iodized salt with mean iodine level of 37.45 ppm [16]. A study in eastern Nepal reported rise in median UIE after iodized salt supplementation [6]. The present median UIE is higher than in the previous study conducted by authors’ team in 2012 in Terai regions of eastern Nepal, where median UIE was reported to be 226.33 μg/L. High iodine intake as shown by increased median UIE can cause iodine induced hyperthyroidism in children [17]. The authors found insufficient UIE in 12.6 % children, which is similar to their previous study results in Terai region, where iodine deficiency was observed in 12.7 % children [17]. The present findings show good improvement in iodine nutrition than shown by National Survey in 2007, which has found 19.4 % children iodine deficient across the whole country [6]. Intricate balance of iodine intake is necessary to maintain optimum iodine nutrition, because both excess and inadequate intake of iodine have harmful effects on thyroid function [17].
The present findings suggest that anemia and iron deficiency are more common in females than males, and in Terai region than hilly region. Anemia and iron deficiency were found in 36.1 % and 48.2 % children respectively in plain district (Udayapur) as compared to 32.7 % and 38 % children respectively in hilly district (Ilam). Dreyfuss et al. reported that high prevalence of hookworms, malaria and vitamin A deficiency are associated with anemia and iron deficiency in the plains (Terai) regions of Nepal [18]. The authors observed iron deficiency in 90.82 % of anemic children, and anemic children had low serum iron and transferrin saturation, and high TIBC. Anemic children had about 13 times more risk of having iron deficiency than non-anemic children. This suggests that iron deficiency is the most common cause for anemia in Nepalese children. Since iron deficiency anemia is the outcome of prolonged iron deficiency, the present finding of majority of iron deficient children (72.26 %) having anemia suggests that a large part of iron deficient population had already suffered from chronic iron deficiency [12]. In Nepal, only 32 % of pre-school children and 29 % of pregnant women were found to consume an adequate amount of iron to fulfill their daily requirements [14]. Globally, the most common cause of anemia is believed to be iron deficiency due to inadequate dietary iron intake, greater physiologic demands and iron losses due to parasitic infections. Other prevalent causes of anemia include malaria, chronic infections and nutritional deficiencies of vitamin A, folate and vitamin B12. The relative contributions of these causes of anemia and iron deficiency vary by sex, age and population and are not well described in many populations including Nepal [18]. While, anemia is the outcome of prolonged iron deficiency, iron deficiency without anemia also has severe effects on child’s physical and mental development [12].
In the present study, the authors observed significant association of UIE with Hb and iron status indicators which has not been reported in earlier studies. They found iodine deficiency to be more common in anemic and iron deficient children than in non-anemic and iron sufficient children. Since, iron is constituent of many enzymes involved in iodine metabolism, coexisting iron deficiency may further deteriorate thyroid hormone synthesis. Iron-deficiency anemia also blunts the efficacy of iodine supplementation [7, 19].
Conclusions
The present study shows high prevalence of anemia and iron deficiency and more than adequate iodine nutrition in Nepalese children. Low urinary iodine excretion was associated with anemia and iron deficiency. Further studies are required to examine the effects of iron deficiency on iodine status in children.
References
Arlappa N, Laxmaiah A, Balakrishna N, et al. Micronutrient deficiency disorders among the rural childrenof West Bengal, India. Ann Hum Biol. 2011;38:281–9.
Diaz JR, de las Cagigas, Rodriguez R. Micronutrient deficiencies in developing and affluent countries. Eur J Clin Nutr. 2003;57:S70–2.
Triggiani V, Tafaro E, Giagulli VA, et al. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr Metab Immune Disord Drug Targets. 2009;9:277–94.
Nepal Demographic and Health Survey 2006. Kathmandu, Nepal: Ministry of Health and Population, New ERA, and Macro International Inc; May 2007.
Khatiwada S, Gelal B, Gautam S, et al. Anemia among school children in eastern Nepal. J Trop Pediatr. 2015. doi:10.1093/tropej/fmv016.
Nepal AK, Khatiwada S, Shakya PR, et al. Iodine status after iodized salt supplementation in school children of eastern Nepal. Southeast Asian J Trop Med Public Health. 2013;44:1072–8.
Zimmermann MB, Kohrle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002;12:867–78.
Bain BJ, Lewis SM, Bates I. Basic haematological techniques. In: Lewis SM, Bain BJ, Bates I, editors. Dacie and Lewis Practical Hematology. 10th ed. Germany. Churchill Livingstone Elsevier; 2006. p. 25–54.
Ohashi T, Yamaki M, Pandav CS, Karmarkar MG, Irie M. Simple microplate method for determination of urinary iodine. Clin Chem. 2000;46:529–36.
Worwood M Iron deficiency anemia and iron overload. In: Lewis SM, Bain BJ, Bates I, editors. Dacie and Lewis Practical Hematology. 10th ed. Germany: Churchill Livingstone Elsevier; 2006. p. 132–60.
World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva: World Health Organization; 2007. viii, 99 p.
Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–20.
Rivera JA, Hotz C, González-Cossío T, Neufeld L, García-Guerra A. The effect of micronutrient deficiencies on child growth: a review of results from community-based supplementation trials. J Nutr. 2003;133:4010S–20.
National Nutrition Policy and Strategy. Kathmandu: Nutrition Section, Child Health Division, Department of Health Services, Ministry of Health and Population, Nepal; 2004 Dec. 58p.
Siegel EH, Stoltzfus RJ, Khatry SK, LeClerq S, Katz J, Tielsch JM. Epidemiology of anemia among 4 to 17 month children living in South Central Nepal. Eur J Clin Nutr. 2006;60:228–35.
Khatiwada S, Gelal B, Tamang MK, Kc R, Singh S, Lamsal M, Baral N. Iodized salt use and salt iodine content among household salts from six districts of eastern Nepal. J Nepal Health Res Counc. 2014;12:191–4.
Khatiwada S, Gelal B, Shakya PR, Lamsal M, Baral N. Urinary iodine excretion among Nepalese school children in terai region. Indian J Pediatr. 2015. doi:10.1007/s12098-015-1755-x.
Dreyfuss ML, Stoltzfus RJ, Shrestha JB, et al. Hookworms, malaria and vitamin a deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J Nutr. 2000;130:2527–36.
Hess SY. The impact of common micronutrient deficiencies on iodine and thyroid metabolism: the evidence from human studies. Best Pract Res Clin Endocrinol Metab. 2010;24:117–32.
Acknowledgments
The authors kindly acknowledge the B P Koirala Institute of Health Sciences (BPKIHS) for providing financial assistance and resources for the study.
Contributions
SK, ML, BG, AKN and NB designed the study. SK, BG and SG performed the field study and laboratory analysis. SK wrote the manuscript. ML, BG, AKN, DB and NB reviewed the manuscript. All authors read and approved the final version of manuscript. NB will act as guarantor for this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
This study was supported by Nepal India Corpus fund through the Institute Advisory Authority of B P Koirala Institute of Health Sciences (BPKIHS).
Rights and permissions
About this article
Cite this article
Khatiwada, S., Lamsal, M., Gelal, B. et al. Anemia, Iron Deficiency and Iodine Deficiency among Nepalese School Children. Indian J Pediatr 83, 617–621 (2016). https://doi.org/10.1007/s12098-015-1924-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-015-1924-y