Abstract
Background
In rectal cancer surgery, larger mesorectal fat area has been shown to correlate with increased intraoperative difficulty. Prior studies were mostly in Asian populations with average body mass indices (BMIs) less than 25 kg/m2. This study aimed to define the relationship between radiological variables on pelvic magnetic resonance imaging (MRI) and intraoperative difficulty in a North American population.
Methods
This is a single-center retrospective cohort study analyzing all patients who underwent low anterior resection (LAR) or transanal total mesorectal excision (TaTME) for stage I–III rectal adenocarcinoma from January 2015 until December 2019. Eleven pelvic magnetic resonance imaging measures were defined a priori according to previous literature and measured in each of the included patients. Operative time in minutes and intraoperative blood loss in milliliters were utilized as the primary indicators of intraoperative difficulty.
Results
Eighty-three patients (39.8% female, mean age: 62.4 ± 11.6 years) met inclusion criteria. The mean BMI of included patients was 29.4 ± 6.2 kg/m2. Mean operative times were 227.2 ± 65.1 min and 340.6 ± 78.7 min for LARs and TaTMEs, respectively. On multivariable analysis including patient, tumor, and MRI factors, increasing posterior mesorectal thickness was significantly associated with increased operative time (p = 0.04). Every 1 cm increase in posterior mesorectal thickness correlated with a 26 min and 6 s increase in operative time. None of the MRI measurements correlated strongly with BMI.
Conclusion
As the number of obese rectal cancer patients continues to expand, strategies aimed at optimizing their surgical management are paramount. While increasing BMI is an important preoperative risk factor, the present study identifies posterior mesorectal thickness on MRI as a reliable and easily measurable parameter to help predict operative difficulty. Ultimately, this may in turn serve as an indicator of which patients would benefit most from pre-operative resources aimed at optimizing operative conditions and postoperative recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The obesity epidemic is a well-known phenomenon in Western society [1]. While obesity is classically associated with cardiovascular disease and insulin resistance, there is also significant evidence supporting an increased risk of colorectal cancer with increasing BMI [2]. Specifically, a body mass index over 30 kg/m2 is associated with a 10–60% increased risk of developing colorectal cancer [3, 4]. As such, patients requiring operative intervention for colorectal cancer have increasingly large BMIs.
The pathophysiological consequences of obesity are complex and pervasive in the perioperative setting [5, 6]. Intraoperatively, obesity is associated with increased operative time and blood loss [7, 8]. Postoperatively, obesity is associated with higher rates of anastomotic leak, surgical site infection (SSI), urinary tract infection (UTI), wound dehiscence, sepsis, and venous thromboembolism (VTE) [9,10,11,12]. Obesity in the setting of surgery for rectal cancer, in particular, carries unique challenges, along with the aforementioned perioperative consequences [12]. Elevated BMI has been associated with difficult total mesorectal excision (TME) in laparoscopic surgery, a key oncological principle to the surgical management of rectal cancer [13, 14].
Obesity has been shown to correlate with increased visceral adiposity bulk and increased mesorectal fat area (MFA) [15, 16]. MFA has been previously identified as a predictor of a difficult TME [8]. In open, laparoscopic, and robotic surgery, larger MFA has correlated with increased operative time and increased intraoperative blood loss [8, 17]. Moreover, MFA, along with other mesorectal radiological measures such as mesorectal-to-pelvis ratio (MPR), have been associated with increased length of stay (LOS) in hospital and postoperative morbidity [8, 18]. However, two of these prior studies were in Asian populations with average BMIs of less than 25 kg/m2, one of which evaluated robotic surgery, thus lacking generalizability to the North American population [8, 18]. A European study by Escal et al. also failed to evaluate a cohort representative of the North American population, as over 80% of the included patients had a BMI of less than 30 kg/m2 [17].
As such, there is currently a paucity of data describing the relationship between mesorectal radiological measures, intraoperative, and postoperative complications in laparoscopic surgery for rectal cancer in North America. The aim of the present retrospective study is to define the relationship between radiological variables on pelvic magnetic resonance imaging (MRI) and intraoperative difficulty. We hypothesize that findings will be similar to previous study demonstrating increasing operative difficulty with increasing MFA. Furthermore, the derivation of an intuitive and easily measurable radiological parameter to identify patients at the greatest risk will be explored in the present study. Ultimately, these associations and measures may help identify patients that are at higher risk of perioperative complication and thus would benefit most from preoperative medical optimization programs.
Materials and methods
Patient selection
The electronic medical records (EMRs) of three minimally invasive colorectal surgeons were searched from January 2015 to December 2019 for patients undergoing elective laparoscopic low anterior resection (LAR) or transanal total mesorectal excision (TaTME) for American Joint Committee on Cancer (AJCC) stage I–III rectal adenocarcinoma. Patients with staging investigations consistent with stage IV disease, patients with prior pelvic colorectal surgery, and patients undergoing palliative resection were excluded. All included patients were over the age of 18, had preoperative staging investigations consistent with stage AJCC I–III rectal adenocarcinoma, and underwent elective, curative laparoscopic surgery. Hamilton Integrated Research Ethics Board approval was obtained for this retrospective study and the need to obtain informed consent was waived. This retrospective cohort study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Surgical technique
All of the included patients underwent laparoscopic tumor-specific TME, defined as complete excision of the visceral mesorectal tissue to a level at least 2 cm distal to the tumor, by one of the three surgeons at St. Joseph’s Healthcare, Hamilton, Ontario, Canada [19]. Choice of surgical approach was dictated by tumor characteristics (i.e., tumor location with respect to anal sphincter complex) and patient characteristics (i.e., comorbidities, preoperative continence, preference, etc.). Care was taken to achieve a high ligation of the inferior mesenteric artery and adequate proximal (> 5 cm) and distal (R0 resection) margins during the course of all operations. Patients with locally advanced disease (i.e., threatened CRM, high-risk stage II disease, stage III disease) and/or a low primary tumor requiring downsizing for attempted sphincter-preserving approach were sent for neoadjuvant therapy. The majority of patients sent for neoadjuvant therapy underwent long course chemoradiotherapy as per local radiation oncologist protocols. No patients underwent total neoadjuvant therapy and all patients underwent surgery between six and 12 weeks following completion of neoadjuvant therapy.
Outcomes assessed
The primary objective of this study was to determine the relationship between preoperative rectal MRI measurements (i.e., MFA, rectal area, mesorectal package area (MPA), bony pelvis area (BPA), lateral mesorectal span (L-MR), anterior–posterior mesorectal span (AP-MR), interspinous distance (IS), AP bony pelvis span (AP-BP), anterior mesorectal thickness (MT), posterior MT) and intraoperative difficulty. Operative time in minutes was utilized as the primary indicator of intraoperative difficulty as in previous studies [8, 20].
Secondary objectives included determining the relationships between BMI and intraoperative difficulty (operative time, intraoperative blood loss, intraoperative anastomotic revision/repair, and nodal harvest) and BMI and preoperative MRI measurements.
Tertiary objectives were aimed at elucidating rates of 30-day mortality and postoperative length of stay (LOS) in days, as well as long-term oncologic outcomes (i.e., overall survival, disease-free survival, local recurrence, distal recurrence). Overall 30-day postoperative morbidity included anastomotic leak, postoperative ileus, high output stoma, superficial surgical site infection (sSSI), intraabdominal abscess, postoperative hemorrhage, wound dehiscence, atelectasis, pneumonia, deep vein thrombosis (DVT), pulmonary embolism (PE), myocardial infarction (MI), myocardial injury following non-cardiac surgery (MINS), urinary tract infection (UTI), acute urinary retention (AUR), acute kidney injury (AKI), reoperation, and readmission.
Data collection
Two study personnel (T.M. and C.K.) as well as an experienced medical administrative assistant abstracted data onto a data collection manual designed a priori on password protected computers and firewall protected programs (RedCap©). Baseline patient characteristics (i.e., age, gender, smoking status, past medical history, past surgical history, medications, allergies) as well as adjuvant therapy information and surgical pathology reports were accessed through the EMRs of the individual surgeons. Preoperative MRIs, MRI radiologist reports, anesthesiologist reports, operative dictations, postoperative discharge summaries, and 30-day follow-up data were accessed through the hospital-based EMR at St. Joseph’s Healthcare Hamilton. Intraoperative complications as well as type of anastomosis (i.e., colorectal, coloanal) and presence or absence of diversion (i.e., loop ileostomy) were extracted from surgical team operative dictations. Postoperative morbidity data were found in discharge summaries and if incomplete, in postoperative daily progress notes.
Pelvic MRI measurements
MRI was performed using either a 1.5 T General Electric (450 W, software version 25) or a 3 T Phillips (Achieva, Software version 3.2.3.5) system. Large field-of-view (FOV) T2-weighted axial images with a slice thickness of 5 mm were downloaded from the PACS system and analyzed with National Institute of Health funded, publicly available software (3D Slicer©, Version 4.11; Bethesda, MD) [21]. A pelvic MRI measurement protocol on 3D Slicer© was developed by a Senior Scientific Research Officer at the Imaging Research Centre at St. Joseph’s Healthcare Hamilton. The protocol was reviewed by an expert radiologist. One study investigator (T.M.) and an independent radiologist (H.J.) were taught the protocol and subsequently completed the pelvic MRI measurements independently on the preoperative MRIs of all of the included patients.
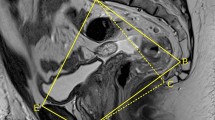
Pelvic MRI measurements were determined using the preoperative MRI closest to the operative date. The MFA has previously been defined as the mesorectal fat at the level of the tip of the ischial spines, as such a single 5 mm slice that most clearly demonstrated the ischial spines was analyzed for each of the included patients [15]. The 11 measures captured in the protocol were divided into mesorectal measurements and pelvic measurements, which are described in Table 1. Linear measures were made using the “ruler” function on 3D Slicer©, which required two consecutive left-mouse clicks with a calibrated cursor at two separate points on the image (Figs. 1, 2, 3). The circumferences of the rectum, mesorectum, and bony pelvis were manually traced with a calibrated cursor to obtain their surface areas (Figs. 4, 5, 6). In areas where a T3 tumor transgressed the rectal wall, thus making muscularis propia difficult to visualize, the trace of the muscularis propria on either side of the tumor was continued along a line following the contour that connected the two visualized edges of the muscularis propria.
Statistical analysis
Descriptive statistics were used to characterize the sample population. Continuous variables were described as means with standard deviations, while categorical variables were reported as numbers and percentages. Statistical significance was set at p < 0.05 and 95% confidence intervals were provided where applicable. One-way ANOVAs, Chi-squared, and Fisher exact tests were used where applicable. Linear regression was performed to estimate associations of MRI variables with operative time. Multivariable analysis of operative time with a multiple linear regression model after assessing for collinearity was then performed. MRI variables with a p < 0.05 on multivariable analysis with MRI variables were entered into a multivariable linear regression analysis of operative time with patient, tumor, and treatment characteristics which were also identified to have a statistically significant association with operative time on univariable linear regression. Variables demonstrating a significant association with main outcomes (i.e., operative time) were considered risk factors. Interobserver agreement for evaluation of pelvic MRI measurements were assessed using a Spearman’s correlation test. Data were analyzed using Stata statistical software (StataCorp, version 15; College Station, TX).
Results
Patient characteristics
In total, 144 patients were identified. Of these, 53 patients were subsequently excluded for not having undergone preoperative pelvic MRI. Additionally, five patients had preoperative MRIs that were not accessible by study personnel. Lastly, three patients were excluded for undergoing multivisceral resection (i.e., concomitant total abdominal hysterectomy, bilateral salpingo-oopherectomy, and rectal resection). As such, 83 consecutive patients (39.8% female, mean age: 62.4 ± 11.6 years) met inclusion criteria and were included in the final analysis. The mean BMI of included patients was 29.4 ± 6.2 kg/m2, with 74.4% of patients having a BMI greater than 24.9 kg/m2 and 43.9% of patients having a BMI of greater than 29.9 kg/m2. The majority of patients (61.5%) had AJCC stage I or II disease. The majority of tumors were T2 or T3 (75.9%), were not threatening the circumferential resection margin (CRM) (84.0%), and had a mean distance of 9.5 ± 4.0 cm from the anal verge. Patients more commonly underwent LAR (69.9%) than TaTME (30.1%). Detailed patient, disease, and operative characteristics are reported in Table 2.
Operative outcomes
Table 3 presents detailed operative outcomes. Operative times were available for 80 (96.4%) patients. Mean operative times were 227.2 ± 65.1 min and 340.6 ± 78.7 min for LARs and TaTMEs, respectively. Operative time was not significantly different between the three surgeons (p = 0.92). Intraoperative blood loss was available for 68 (81.9%) patients. Mean blood loss was 148.1 ± 82.0 mL for patients undergoing LAR and 247.9 ± 282.6 mL for patients undergoing TaTME. Overall, 30-day postoperative morbidity was 45.8%, with the most common complications being postoperative ileus (n = 18, 21.7%), anastomotic leak (n = 12, 14.5%), and AUR (n = 9, 10.8%). There was no 30-day postoperative mortality. Median postoperative LOS following index surgery was 5 days (range: 2–63 days). Sixty-eight patients had pathology reports commenting on completeness of TME, 13 of which had incomplete TME (19.1%). TME completeness was not significantly associated with BMI nor any of the pelvic MRI measures. Oncologic outcomes beyond 30 days postoperatively included 3.4% mortality, 2.0% local recurrence, and 7.3% distant recurrence. Mean follow-up was 2.3 years.
Factors associated with operative time
The associations between operative time in quartiles and patient characteristics, tumor characteristic, disease characteristics, intraoperative morbidity, and postoperative morbidity are presented in Table 4. Greater BMI was the only patient characteristic significantly associated with increasing operative time (p = 0.03). Shorter distance from the anal verge was the only tumor characteristic associated with increasing operative time (p = 0.001). Patients subjected to longer operative times were more likely to have undergone TaTME (p < 0.001). Increased intraoperative blood loss (p = 0.04) was associated with significantly increased operative time. Postoperative morbidity was associated with longer operative time (p < 0.001).
MRI characteristics
Mean mesorectal and pelvic MRI measures are reported in Table 5. None of the MRI measurements correlated strongly with BMI. AP-BP had the strongest positive correlation with BMI (r = 0.47) and L-MR had the strongest negative correlation with BMI (r = − 0.13). Total MT (r = 0.30), anterior MT (r = 0.27), and posterior MT (r = 0.19) demonstrated weak positive correlations with increasing BMI. Interobserver variations in MRI measurements are summarized in Supplemental Table 1. The Spearman’s correlation coefficients ranged from 0.74 to 0.99 (p < 0.05 for all measures).
Operative times and MRI measurements
Univariable analysis of MRI mesorectal and pelvic measures demonstrated that larger AP-MR, posterior MT, Total MT, MPA, MFA, and AP-BP were significantly associated with increased operative time (Table 6). Upon removing collinear variables, multivariable analysis of MRI measures showed larger posterior MT and AP-BP were significantly associated with increased operative time (Table 6). On multivariable analysis including patient, tumor, and MRI factors, greater posterior MT remained significantly associated with increased operative time (p = 0.04). For every 1 mm increase in posterior MT, a 2 min and 6 s increase in operative time was noted (Table 7). Other factors significantly associated with increased operative time on multivariable analysis included male gender (p = 0.05), greater BMI (p = 0.04), and shorter tumor distance from the anal verge (p = 0.006). Increased postoperative morbidity was both associated with significantly increased operative time (p < 0.001).
Discussion
The obesity epidemic has a myriad of health-related consequences [22]. From a rectal cancer perspective, not only does obesity increase the risk of developing rectal cancer, but it also increases the technical difficulty of its surgical management [2, 20]. In an attempt to more accurately quantify the intraoperative difficulty associated with obesity in rectal cancer surgery, obesity has been correlated with MFA. Increasing MFA has been demonstrated to reflect increases in BMI, as well as predict increased operative time and intraoperative blood loss [8, 16, 17]. Nonetheless, there remains little data describing the relationship between mesorectal radiological measures and intraoperative complications in laparoscopic surgery for rectal cancer in North America. As such, the present study examined the relationship between 11 rectal MRI measurements and intraoperative difficulty, as measured by operative time and intraoperative blood loss, in a cohort of 83 patients who underwent laparoscopic LAR or TaTME between January 2015 and December 2019. Posterior mesorectal thickness was found to be a significant predictor of increased operative time (p = 0.04) and increased intraoperative blood loss (p = 0.001). A 1 cm increase in posterior mesorectal thickness was associated with a 26 min and 6 s increase in operative time and a 118.7 mL increase in intraoperative blood loss. Male gender (p = 0.05), greater BMI (p = 0.04), and shorter tumor distance from the anal verge (p = 0.006) were also associated with significantly increased operative time. Interestingly, BMI did not correlate strongly with any mesorectal or pelvic MRI measurements.
Posterior mesorectal thickness is a simple and intuitive measure that was defined as the linear distance from the most posterior aspect of the muscularis propria of the rectum to the most posterior aspect of the mesorectal fascia. Previous studies have demonstrated higher rates of intraoperative and postoperative morbidity with increasing MFA and decreasing bony pelvis measures, but none have measured posterior mesorectal thickness [8, 17, 23]. The majority of the mesorectal fat is distributed posterior to the rectum; therefore, perhaps, the measurement of posterior mesorectal thickness more accurately encompasses mesorectal bulk [24]. Intraoperatively, the posterior mesorectal fascial plane is often the first to be incised [25]. Greater posterior mesorectal thickness may increase the amount of time and dissection required to identify the correct anatomical plane, as well as increase the difficulty of maintaining adequate retraction anteriorly for visualization of the deep pelvic planes [26]. As the procedure advances caudally, careful dissection of the posterior plane is of the utmost importance as breaching the mesorectal fascia anteriorly can increase the risk of local recurrence and breaching the presacral fascia posteriorly can damage sacral nerves as well as the presacral plexus [27]. Disruption of the presacral plane in particular may lead to intraoperative bleeding [28]. Altogether, a larger posterior mesorectum potentially increases the technical difficulty associated with these aspects of a TME given the significant association with increased operative time and intraoperative blood loss in the present study. In contrast to previously developed pelvimetric scores to predict intraoperative difficulty, posterior mesorectal thickness is a single measure easily obtainable on any MRI or computed tomography (CT) software [17, 29]. Therefore, consideration to highlighting this value on preoperative MRI or CT reports may help surgeons better predict operative difficulty and plan operations accordingly.
Considering the impact of posterior mesorectal thickness on operative time and intraoperative blood loss, it may also have the potential to be an important modifiable risk factor in rectal cancer patients. Current pre-habilitation programs for colorectal cancer patients are aimed at optimizing patients’ functional and nutritional statuses in the weeks prior to surgery [30, 31]. While these are important factors, younger obese colorectal patients may derive greater benefit from pre-habilitation strategies geared toward weight loss and decreasing visceral adiposity [10]. For example, a four-week course of a very low energy diet (VLED) with Optifast© significantly decreased mesorectal fat volume on MRI in a cohort of bariatric surgery patients [32]. Employing pre-operative VLEDs in obese rectal cancer patients could have the same benefit, thus decreasing posterior mesorectal thickness, and potentially improving intraoperative and postoperative outcomes. The current study in this area is underway (NCT0437930; ACTRN12615000941561). Moreover, posterior mesorectal thickness may be an important variable to consider during the preoperative planning phase. If demonstrated on preoperative MRI, extensive posterior mesorectal thickness could prompt consideration for booking a longer operative time or relying on advanced laparoscopic techniques, such as robotic-assisted surgery. Robotic-assisted TME is less likely to be impacted by difficult pelvic anatomy and allows more degrees of freedom during dissection within the confines of a narrow bony pelvis [33, 34].
Increasing anterior bony pelvis span, defined as the linear distance from the most posterior aspect of the symphysis pubis anteriorly to the most anterior aspect of the coccyx posteriorly, also correlated significantly with increased operative time (p = 0.05). This is somewhat counterintuitive given the assumption that a larger bony pelvis structure provides increased operative domain and thus decreased technical difficulty. However, previous study has more consistently highlighted increasing pelvic width, not anterior–posterior span, as an indicator of decreased intraoperative difficulty [35, 36]. Moreover, anterior–posterior pelvis span demonstrated the strongest correlation with BMI (r = 0.47) and thus may be reflective of the association between increasing operative time and increasing BMI [7, 8, 37].
This study has several limitations. First, there were only 83 patients that met inclusion criteria. This limited statistical power and thus other significant predictors of increased operative time may not have been identified. For example, in a previous study including 98 patients, MFA was identified as a significant predictor of operative time, but in the present study, this was not found (p = 0.75) [8]. Moreover, the low number of included patients limited the number of postoperative complications observed (n = 46) and precluded adequately powered statistical analysis aimed at determining the relationship between mesorectal and pelvic MRI measures and postoperative outcomes. Second, missing data from surgeon and institutional EMRs further limited statistical analysis. For example, intraoperative blood loss was only recorded for 81.9% of included patients. Furthermore, pre-neoadjuvant therapy MRIs were analyzed for patients in which post-neoadjuvant therapy MRIs were not available. While there are no data to suggest significant changes in mesorectal measures due to neoadjuvant radiotherapy, it may have introduced heterogeneity to the population of analyzed MRIs [38]. Third, the single-center, retrospective nature of the present study increases the risk of bias of the observed results and decreases generalizability. However, patient demographics and treatment outcomes align with previously reported North American data and offer reassurance as to the generalizability of the observed results [39, 40]. Nonetheless, there remain multiple possible confounders that are important to consider when interpreting the results of the present study, such as nuanced operative techniques (e.g., splenic flexure mobilization, type of anastomosis) and patient variables (e.g., BMI, gender, prior pelvic operations, or radiation). Fourth, including patients undergoing LARs and TaTMEs introduced heterogeneity into the study population and may have slightly decreased the applicability of the effect estimate derived from our data. Despite TaTMEs having significantly longer operative times than LARs, there are no current data suggesting that mesorectal measures differ between patients undergoing LAR and TaTME. Fifth, 3D Slicer© is not a standard component of the MRI workstation and thus its use limits generalizability of some of the MRI measurements. Nonetheless, the mesorectal measurement that correlated best with operative difficulty, posterior mesorectal thickness, is a simple linear measurement that can be completed with the standard “ruler” function available on standard Picture and Archiving Communication Systems (PACS). Lastly, total operative time was used as a surrogate measure for intraoperative difficulty. Previous studies have suggested that pelvic dissection times are a more accurate reflection of the impact of mesorectal bulk in rectal cancer surgery; however, these data were not available through the St. Joseph’s Healthcare Hamilton EMR [8]. Nonetheless, major factors influencing total operative time, such as multi-visceral resections and conversion to open procedure, were excluded from the present study.
As the number of obese rectal cancer patients continues to expand, strategies aimed at optimizing the surgical management of this challenging patient population are paramount. Identifying which patients will present operative difficulty is the first step in optimizing management. While greater BMI is an important preoperative risk factor, the present study identifies posterior mesorectal thickness on MRI as a reliable and easily measurable parameter to predict whether patients may benefit from perioperative interventions to improve operative conditions. Specifically, this study suggests that male patients with low rectal tumors, elevated BMI, and large posterior mesorectums pose heightened intraoperative difficulty as measured by operative time. These patients should be considered for enhanced laparoscopic approaches (e.g., robotic-assisted surgery), pre-operative VLED, or be booked for longer operative duration with high-level assistance in the operating room. Future work should aim to validate these findings prospectively as well as identify the impact of decreasing mesorectal bulk through pre-habilitation interventions such as VLED.
References
WHO. Obesity and Overweight.; 2016. http://www.who.int/mediacentre/factsheets/fs311/ en/2016. Accessed November 1, 2019.
Moghaddam AA, Woodward M, Huxley R (2007) Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 16(12):2533–2547. https://doi.org/10.1158/1055-9965.EPI-07-0708
Bianchini F, Kaaks R, Vainio H (2002) Review Overweight, obesity, and cancer risk 3(September):565–574
Larsson SC, Wolk A (2007) Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am J Clin Nutr 86(3):556–565. https://doi.org/10.1093/ajcn/86.3.556
Kabon B, Nagele A, Reddy D et al (2004) Obesity decreases perioperative tissue oxygenation. Anesthesiology 100(2):274–280. https://doi.org/10.1097/00000542-200402000-00015
Toma O, Suntrup P, Stefanescu A, London A, Mutch M, Kharasch E (2011) Pharmacokinetics and tissue penetration of cefoxitin in obesity: implications for risk of surgical site infection. Anesth Analg 113(4):730–737
Ri M, Aikou S, Seto Y (2018) Obesity as a surgical risk factor. Ann Gastroenterol Surg 2(1):13–21. https://doi.org/10.1002/ags3.12049
Yamaoka Y, Yamaguchi T, Kinugasa Y et al (2019) Mesorectal fat area as a useful predictor of the difficulty of robotic-assisted laparoscopic total mesorectal excision for rectal cancer. Surg Endosc 33(2):557–566. https://doi.org/10.1007/s00464-018-6331-9
Geiger TM, Muldoon R (2011) Complications following colon rectal surgery in the obese patient. Clin Colon Rectal Surg 24(4):274–282. https://doi.org/10.1055/s-0031-1295692
Wahl TS, Patel FC, Goss LE, Chu DI, Grams J, Morris MS (2018) The obese colorectal surgery patient: Surgical site infection and outcomes. Dis Colon Rectum 61(8):938–945. https://doi.org/10.1097/DCR.0000000000001085
Tjeertes EEKM, Hoeks SSE, Beks SSBJC, Valentijn TTM, Hoofwijk AAGM, Stolker RJRJ (2015) Obesity - a risk factor for postoperative complications in general surgery? BMC Anesthesiol 15(1):1–7. https://doi.org/10.1186/s12871-015-0096-7
Qiu Y, Liu Q, Chen G et al (2016) Outcome of rectal cancer surgery in obese and nonobese patients: a meta-analysis. World J Surg Oncol 14(1):1–7. https://doi.org/10.1186/s12957-016-0775-y
Zhou XC, Su M, Hu KQ et al (2016) CT pelvimetry and clinicopathological parameters in evaluation of the technical difficulties in performing open rectal surgery for mid-low rectal cancer. Oncol Lett 11(1):31–38. https://doi.org/10.3892/ol.2015.3827
Chen J-H, Andrews JM, Kariyawasam V et al (2016) Review article: acute severe ulcerative colitis - evidence-based consensus statements. Aliment Pharmacol Ther 44(2):127–144. https://doi.org/10.1111/apt.13670
Boyle KM, Chalmers AG, Finan PJ, Sagar PM, Burke D (2009) Morphology of the mesorectum in patients with primary rectal cancer. Dis Colon Rectum 52(6):1122–1129. https://doi.org/10.1007/DCR.0b013e31819ef62f
Allen SD, Gada V, Blunt DM (2007) Variation of mesorectal volume with abdominal fat volume in patients with rectal carcinoma: Assessment with MRI. Br J Radiol 80(952):242–247. https://doi.org/10.1259/bjr/66311683
Escal L, Nougaret S, Guiu B et al (2018) MRI-based score to predict surgical difficulty in patients with rectal cancer. Br J Surg 105(1):140–146. https://doi.org/10.1002/bjs.10642
Chen B, Zhang Y, Zhao S et al (2016) The impact of general/visceral obesity on completion of mesorectum and perioperative outcomes of laparoscopic TME for rectal cancer A STARD-compliant article. Med (United States) 95(36):e4462. https://doi.org/10.1097/MD.0000000000004462
Lowry AC, Simmang CL, Boulos P et al (2001) Consensus statement of definitions for anorectal physiology and rectal cancer: Report of the tripartite consensus conference on definitions for anorectal physiology and rectal cancer, Washington, DC, May 1, 1999. Dis Colon Rectum 44(7):915–919. https://doi.org/10.1007/BF02235475
Chen W, Li Q, Fan Y et al (2016) Factors predicting difficulty of laparoscopic low anterior resection for rectal cancer with total mesorectal excision and double stapling technique. PLoS ONE 11(3):1–13. https://doi.org/10.1371/journal.pone.0151773
Fedorov A, Beichel R, Kalpathy-Cramer J et al (2012) 3D slicer as an image computing platform for teh quantitative imaging network. Magn Reson Imaging 30(9):1323–1241
Després J-P (2001) Health consequences of visceral obesity. Ann Med 33(8):534–541
Curtis NJ, Thomas C, Dennison G et al (2019) Factors predicting operative difficulty of laparoscopic total mesorectal excision. Dis Colon Rectum 62(12):1467–1476. https://doi.org/10.1097/DCR.0000000000001490
Kulaylat MN (2015) Mesorectal excision: surgical anatomy of the rectum, mesorectum, and pelvic fascia and nerves and clinical relevance. World J Surg Proced 5(1):27. https://doi.org/10.5412/wjsp.v5.i1.27
Wibe A, Møller B, Norstein J et al (2002) A national strategic change in treatment policy for rectal cancer - implementation of total mesorectal excision as routine treatment in Norway. A national audit Dis Colon Rectum 45(7):857–866. https://doi.org/10.1007/s10350-004-6317-7
Lichliter WE (2015) Techniques in total MESORECTAL excision surgery. Clin Colon Rectal Surg 28(1):21–27. https://doi.org/10.1055/s-0035-1545066
Diop M, Parratte B, Tatu L, Vuillier F, Brunelle S, Monnier G (2003) “Mesorectum” The surgical value of an anatomical approach. Surg Radiol Anat 25(3–4):290–304. https://doi.org/10.1007/s00276-003-0148-4
Lou Z, Zhang W, Meng RG, Fu CG (2013) Massive presacral bleeding during rectal surgery: From anatomy to clinical practice. World J Gastroenterol 19(25):4039–4044. https://doi.org/10.3748/wjg.v19.i25.4039
Ferko A, Malý O, Örhalmi J, Dolejš J (2016) CT/MRI pelvimetry as a useful tool when selecting patients with rectal cancer for transanal total mesorectal excision. Surg Endosc 30(3):1164–1171. https://doi.org/10.1007/s00464-015-4324-5
Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJE (2019) Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg 43(7):1661–1668. https://doi.org/10.1007/s00268-019-04950-y
Bolshinsky V, Li MHG, Ismail H, Burbury K, Riedel B, Heriot A (2018) Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Dis Colon Rectum 61(1):124–138. https://doi.org/10.1097/DCR.0000000000000987
Bell S, Malouf P, Johnson N et al (2019) Pelvic fat volume reduction with preoperative very low energy diet (VLED): implications for rectal cancer surgery in the obese. Tech Coloproctol 23(9):887–892. https://doi.org/10.1007/s10151-019-02074-y
Baik SH, Kang CM, Lee WJ et al (2007) Robotic total mesorectal excision for the treatment of rectal cancer. J Robot Surg 1(1):99–102. https://doi.org/10.1007/s11701-007-0015-0
Baek SJ, Kim CH, Cho MS et al (2015) Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg Endosc 29(6):1419–1424. https://doi.org/10.1007/s00464-014-3818-x
Salerno G, Daniels IR, Brown G, Heald RJ, Moran BJ (2006) Magnetic resonance imaging pelvimetry in 186 patients with rectal cancer confirms an overlap in pelvic size between males and females. Color Dis 8(9):772–776. https://doi.org/10.1111/j.1463-1318.2006.01090.x
Hyuk Baik S, Kyu Kim N, Young Lee K et al (2008) Factors influencing pathologic results after total mesorectal excision for rectal cancer: Analysis of consecutive 100 cases. Ann Surg Oncol 15(3):721–728. https://doi.org/10.1245/s10434-007-9706-z
Blee TH, Belzer GE, Lambert PJ (2002) Obesity: Is there an increase in perioperative complications in those undergoing elective colon and rectal resection for carcinoma? Am Surg 68(2):163–166
Koh D, Chau I, Tait D, Wotherspoon A, Cunningham D, Brown G (2008) Evaluating mesorectal lymph nodes in rectal cancer before and after neoadjuvant chemoradiation using thin-section T2-weighted magnetic resonance imaging. Int J Radiat Oncol Biol Phys 71(2):456–461
Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Based on November 2019 SEER data submission, posted to the SEER web site, April 2020. https://seer.cancer.gov/csr/1975_2017/.
Vergara-Fernandez O, Swallow CJ, Victor JC et al (2010) Assessing outcomes following surgery for colorectal cancer using quality of Care indicators. Can J Surg 53(4):232–240
Acknowledgements
We thank Ms. Sara McKillop, an experienced medical administrative assistant, for her assistance in conducting the retrospective electronic medical records review. We thank Mr. Norm Konyer, Senior Scientific Research Officer at St. Joseph’s Healthcare Hamilton Imaging Research Centre, for his assistance in developing and teaching the MRI measurement protocol with 3D Slicer© (Version 4.11; Bethesda, MD).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Study concept and design—Ramji, McKechnie, Noseworthy, Rebello, Eskicioglu. Acquisition of data—Ramji, McKechnie, Kruse. Analysis and interpretation of data—All authors. Drafting of manuscript—Ramji, McKechnie, Doumouras. Critical revision of the manuscript for intellectual content—All authors. Final approval of version to be published—All authors.
Corresponding author
Ethics declarations
Disclosures
Dr. Eskicioglu, Dr. Hong, Dr. Doumouras, Dr. Amin, Dr. Cadeddu, Dr. Rebello, Dr. Noseworthy, Mr. Kruse, Dr. Ramji, and Dr. McKechnie do not have proprietary or commercial interest in any product mentioned or concept discussed in this article to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been accepted as a Presentation on Demand at the American Society of Colon and Rectum Surgeons Annual Scientific Meeting in San Diego, CA (Apr. 24-28th, 2021).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McKechnie, T., Ramji, K., Kruse, C. et al. Posterior mesorectal thickness as a predictor of increased operative time in rectal cancer surgery: a retrospective cohort study. Surg Endosc 36, 3520–3532 (2022). https://doi.org/10.1007/s00464-021-08674-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08674-w