Abstract
Background
In right-sided colon cancer surgery, currently there is a great deal of discussion and debate regarding complete mesocolic excision (CME) versus conventional right hemicolectomy (CRH) on postoperative outcomes and oncological results.
Our aim was to perform a systematic review of the short- and long-term outcomes of CME to standardize surgical approach in patients with right-sided colon cancer.
Methods
A systematic review was performed examining available data on randomized and non-randomized studies evaluating the role of CME and D3 lymphadenectomy in the treatment of right-sided colon cancer, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards.
Results
After literature search, 919 studies have been recorded, 110 studies underwent full-text reviews and 30 studies met inclusion criteria. The total number of CME procedures was 5931. Postoperative complications was reported in 28 studies with pooled overall complications of 1.88% for CME surgery. Six studies reported 0% of overall postoperative complications and they demonstrated a low incidence of complications following CME procedure. Anastomotic leak was reported in 27 studies with pooled proportion of 0.92% after CME resections. There were 16 papers reporting overall survival following CME procedure, with a mean of 85% of patients survived at 5 years. Mean 5-year overall survival was 93.05% in stage I patients, 89.76% in stage II patients and 79.65% in stage III patients. Local and distant recurrence were included in 21 studies, reporting tumor recurrence rate of 12.25% following CME. 5-year tumor recurrence was 5.8% in stage I patients, 7.68% in stage II patients and 15.69% in stage III patients.
Conclusions
CME does not increase the risk of postoperative complications and significantly improves the long-term oncological impact. Prospective multicentre studies results are needed to verify if CME could be considered standard surgery for right colon cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Oncological radicality is an essential concept in colon cancer surgery where upfront surgical resection in combination with selective use of adjuvant chemotherapy are main modalities of treatment. Just as rectal cancer surgery has been revolutionized after introduction of total mesorectal excision (TME) by Heald et al. [1], so as complete mesocolic excision (CME) and the extended lymphadenectomy along the mesenteric axis with central vascular ligation (CVL) of the inferior mesenteric vessels is standard in left hemicolectomy for colon cancer. However, in right-sided colon cancer surgery, currently there is a great deal of discussion and debate regarding CME versus conventional right hemicolectomy (CRH) on postoperative outcomes and oncological results.
Although clear definition of CRH is lacking in literature, it could be defined as the associated resection of the terminal portion of the ileum, the ascending colon, the proximal transverse portion and the entire lymph node drainage area on the right edge of the superior mesenteric axis. In 2009 Hohenberger et al. [2] firstly described CME aiming at the separation of the visceral plane from the parietal one, lymph node dissection, central ligation of the supplying vessels (CVL) and, if any extra-colonic organs or structures were attached to the tumor, multivisceral resection extending the dissection plane to the next embryologic plane beyond the involved organ or structure not invaded, performed in an en bloc fashion. By consequent application of the procedure of CME, the study describes the reduction of local 5-year recurrence rates in colon cancer from 6.5 to 3.6% compared to patients who received a conventional resection. In the same period, the cancer related 5-year survival rates in patients resected for cure increased from 82.1 to 89.1%.
Japanese D3 lymphadenectomy has been performed in many Asian countries which is based on similar principles to CME with CVL, according to the Japanese Society for Cancer of the Colon and Rectum [3]. When comparing D3 specimens with CME specimens, both specimens showed higher rates of the mesocolic plane surgery and long distances from the high vascular tie to the bowel wall [4]. CME surgery increased the number of nodes retrieved compared with non-CME surgery [5,6,7]. Nevertheless, CME is technically demanding and several authors [8] have been associated it with more intraoperative organ injuries and severe non-surgical complications compared to CRH.
The aim of this study is to perform a systematic review of the short- and long-term outcomes of CME to standardize surgical approach in patients with right-sided colon cancer.
Materials and methods
Search strategy
A systematic review was performed examining available data on randomized and non-randomized studies evaluating the role of complete mesocolic excision (CME) and D3 lymphadenectomy in the treatment of right-sided colon cancer, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards (Table 1) [9]. Eligible studies were identified using 3 distinct databases through January 2020: Medline (PubMed), Cochrane library and Scopus. The following terms have been used for the research: “complete mesocolic excision”, combined with “colon cancer”, “D3 lymphadenectomy” and “right colectomy”, without any language or publication restrictions. Full-text papers were independently screened by two authors (G.M. and E.M.M.) for eligibility. Reference lists of eligible studies were assessed manually so that no relevant article was missed.
Inclusion and exclusion criteria
In the systematic review, we restricted the search using the following exclusion criteria: (1) animal studies, (2) studies involving TNM stage IV; studies involving other tumor sites besides the right-sided localization; (3) reviews and meta-analyses, (4) editorials and letters to the editors or case series with less than ten treated patients. Only studies with significant data on patients undergoing CME for right-sided colon cancer (involving proximal transverse location) have been included.
Data extraction and synthesis
After reviewing the full-texts of eligible studies, 2 authors (G.M., E.M.M.) performed the data extraction and cross-checked all results. Extracted variables included: general study characteristics, patient demographics, TNM staging, postoperative outcomes and oncological results. General study characteristics included author, journal, year of publication, study design, number of CME patients and tumor localization; patient demographics included age, American Society of Anesthesiology (ASA) score and body mass index (BMI); staging of tumor included TNM stage I,II and III; finally, postoperative outcomes included complications (anastomotic leakage, gastroplegia, prolonged postoperative ileus and reoperation rate) and oncological results (5-year tumor recurrence and 5-year overall survival). When coding the data, any disagreements were adjudicated by a third reviewer (B.P.). Data were tabulated and cumulative analysis was performed when possible. Categorical variables were extracted as numbers and reported as proportions, tumor recurrence (TR) was defined as the time from CME surgical procedure treatment to metastatic or locoregional disease recurrence. Overall survival (OS) was defined as the time from CME surgical procedure treatment to death or last follow-up.
Results
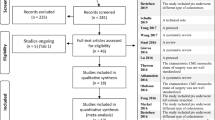
The results of the systematic review are presented in the PRISMA flow chart in Fig. 1. After literature search, 919 studies have been recorded, 110 studies underwent full-text reviews and 30 studies met inclusion criteria. Study design and characteristics are reported in Table 1. Of the 30 included studies, 23 studies were retrospective, 5 were prospective and 2 were randomized controlled trials (RCTs) published from 2009 to 2020.
In our systematic review the total number of CME procedures was 5931 and the sample size ranged from 17 to 1366. 21 studies (patients n = 2616) fully reported minimally invasive surgery (MIS) (15 laparoscopic, 4 robotic and 2 both laparoscopic and robotic CME); 7 studies (patients n = 2876) described open and laparoscopic CME surgery; only 2 studies (patients n = 439) reported open technique. All studies (100%) included right-sided resections. Patient demographics: only 8 studies analyzed patients with a mean age over 70 years old [11, 14,15,16, 18, 25, 28, 29], 1 study does not specify the sample mean age [34] and the remainder performed CME procedures in a mean age of patients under 70 years old; 22 studies analyzed patients with a mean BMI under 30 [11, 13, 14, 16,17,18, 21,22,23,24,25,26,27,28, 31, 32, 34,35,36,37,38,39], only 1 study reported a mean BMI of 30 [29] and the remainder did not specify the BMI of patients underwent CME; there is no prevalence of ASA score among patients in all studies analyzed.
According to the Union for International Cancer Control (UICC), all studies reported every pathological T and N stage, one study included only T1-T3 [26] and one study included only N + stage [37].
Postoperative outcomes
Postoperative complications was reported in 28 studies (patients n = 4862) (Table 2) with pooled overall complications included in the research of 1.88% for CME surgery [10, 12,13,14,15,16,17,18,19,20,21,22,23,24, 26,27,28,29,30,31,32,33,34,35,36,37,38,39]. In the higher volume study (patients n = 1366) [17], the pooled overall incidence of complications was 3.65% and 5.8% in lower volume study (patients n = 17) [15].
Anastomotic leak was reported in 27 studies (patients n = 4793) of this systematic review with pooled proportion of 0.92% after CME resections [12,13,14,15,16,17,18,19,20,21,22,23,24, 26,27,28,29,30,31,32,33,34,35,36,37,38,39]. In the higher volume study (patients n = 1366) [17], the anastomotic leak rate was 1.3 and 0% in lower volume study (patients n = 17) [15]. 12 studies (patients n = 729) reported 0% of anastomotic leak rate in their series of CME procedures performed [13, 15, 18, 20, 22, 24, 26, 32,33,34,35,36].
Prolonged postoperative ileus was reported in 20 studies (patients n = 3737) with pooled proportion of 4.71% [14,15,16,17,18, 21,22,23,24, 27, 28, 30, 31, 33,34,35,36,37,38,39]. In the higher volume study (patients n = 1366) [17], the postoperative ileus rate was 6% and 17.6% in lower volume study (patients n = 17) [15]. 4 studies (patients n = 232) reported 0% of prolonged postoperative ileus rate in their series of CME procedures performed [18, 27, 33, 34].
Gastroplegia is the less reported complication among studies in this systematic review.
Finally, overall reoperation rate was reported in 18 studies (patients n = 2268) with a pooled proportion of 1.51% [10, 13,14,15,16, 18, 19, 21, 23, 28, 29, 32,33,34,35,36, 38, 39], and 0% in 8 studies (patients n = 651) included in this review [15, 18, 32,33,34,35,36, 38].
Oncological results
Overall oncological results are reported in Table 3, UICC stage-based OS and TR are listed in Tables 4 and 5.
In our systematic review, there were 16 papers (patients n = 4625) reporting OS following CME procedure [10,11,12, 14, 17, 19,20,21,22,23,24,25, 28,29,30,31], with a mean of 85% of patients (n = 3865) survived at 5 years. In the higher volume study (patients n = 1366) [17], OS was 89.2%. In lower volume study (patients n = 34) [20], OS was 100%. Oncological results with available data divided for each stage (I, II and III) were collected where possible. There were some patients without data from the OS after CME. Mean 5-year OS was 93.05% in stage I patients (n = 358), 89.76% in stage II patients (n = 775) and 79.65% in stage III patients (n = 683). In our systematic review, three studies reported 100% OS in stage I patients [10, 20, 31], one study reported 100% OS in stage II patients [20] and one study reported 100% OS in stage III patients underwent CME [20].
Local and distant recurrence was included in 21 studies (patients n = 4900), reporting TR rate of 12.25% following CME [10,11,12,13,14,15,16,17, 19,20,21,22,23,24,25, 28,29,30,31, 35, 36]. In the higher volume study (patients n = 1366) [17], TR was 13.4%. In lower volume study (patients n = 34) [20], TR was 0%. 5-year TR was 5.8% in stage I patients, 7.68% in stage II patients and 15.69% in stage III patients. In our systematic review, five studies reported 0% TR in stage I patients [10, 11, 13, 15, 28], two studies reported 0% TR in stage II patients [13, 15] and two studies reported 0% TR in stage III patients underwent CME [13, 15].
Discussion
Complete mesocolic excision (CME)
The CME procedure involves the principle of sharp anatomic dissection along the embryologic planes with preservation of an intact visceral fascia of the mesocolon analogous to the concept of total mesorectal excision with sharp dissection along the fascia propria of the mesorectum [40]. If the cancer is located in the right colon the surgical procedure includes mobilization of the duodenum with the pancreatic head (Kocher maneuver) and the mesenteric root up to the origin of the superior mesenteric artery for optimal exposure of the supplying vessels. The attachments of the mesenteric plane covering the duodenum and the uncinate process were taken down from the corresponding one of the mesenteric root to get full access to the superior mesenteric vein and the artery behind [2].
D3 lymphadenectomy
Resection of the D1 lymph node represents transection of the mesenteric vessels at the level proximal to the marginal vessel, whereas D2 resection is a more traditional resection of the main feeding vessel to a given colonic segment at its origin. Dissection of D2 should include vascular ligation and lymphadenectomy that includes the origin of the named feeding vessel (e.g., ileocolic artery at its origin from the superior mesenteric artery or superior rectal artery at the takeoff of the left colic artery). Dissection of D3 represents an extended lymphadenectomy that includes lymph nodes along the root vessel. A D3 dissection for a right-sided tumor includes lymph nodes along the anterior aspect of the superior mesenteric vein (SMV) and superior mesenteric artery (SMA) (central lymph nodes) and for a left sided tumor includes lymph nodes around the inferior mesenteric artery (IMA). [2, 3] However, in addition, for cancer of the hepatic flexure of the colon, about 5% positive lymph nodes can be found over the head of the pancreas, and less frequently (4%) [41], in lymph nodes along the gastroepiploic arcade at the greater curvature of the stomach [2].
Central vascular ligation (CVL)
Following the complete mobilization of the right colon including the mesenteric root, the entire bowel can easily be twisted in a clockwise fashion to achieve easy access to the central part of the superior mesenteric vein and artery [42].
Hohenberger et al. [2] proposed technical notes on nodal dissection, noted as CVL. According to the pattern of potential lymphatic spread, first the ileocolic and if present the right colic vessels were divided at their origin from the SMV and SMA, respectively. For cancer of the caecum and ascending colon, only the right branches of the middle colic vessels are divided centrally. So, the colon was divided right at the level of the middle colic vessels. Cancer of the transverse colon including both flexures needs true central ligation of the middle colic artery and vein considering the variations that may be found. In addition, according to the additional pattern of lymphatic spread described above, central tie of the right gastroepiploic artery and vein may be needed.
Preservation of the surrounding autonomous nervous plexus was ensured to avoid the risk of functional sequelae, e.g. diarrhea. If lymph nodes over the pancreatic head were potentially involved, these nodes were dissected off the pancreatic head with central ligation of the right gastroepiploic artery. The superior pancreaticoduodenal artery was usually preserved.
Classically, CVL includes individual proximal vascular ligation, with extended central lymph node dissection. The benefits conferred by this type of extended resection need to be interpreted in the context of the increased surgical complications that can occur [40].
Colorectal cancer is one of the most common malignant tumors in western countries. Worldwide approximately more than one million people per year develop this tumor and more than half of them will die from this malignancy [43].
Currently, matter of debate is the potential oncologic impact and the risk–benefit ratio of CME compared with CRH. Objective of this systematic review was to investigate surgical safety, the short- and long-term outcomes of CME in patients with right colon cancer.
Several authors have reported some comparative reviews showing improved recurrence rates and survival from CME compared to conventional surgery [44,45,46].
However, more accurate results may be available in some updated meta-analyses [47,48,49,50,51], aiming to assess the comparative oncological benefits of CME. In the meta-analyses by Chao Wang et al. [47], CME had positive effects on survival for stage III disease compared with CRH. Furthermore, Ow ZGW et al. [48] published the first systematic review and meta-analysis to demonstrate that CME and D3 lymphadenectomy has superior long-term survival outcomes compared to CRH and D2 lymphadenectomy, with similar complications rates in the two groups. The most recent meta-analysis reports that rates of local and distant recurrence were lower in the CME group, as well as pooled 5‐year overall survival was significantly higher [49]. Other authors [50], conclude that its safety and survival benefits need to be further studied.
To our knowledge, only another review and meta-analysis in the literature exclusively analyzes the outcomes of the CME in the right-sided colon cancer [51], showing that no difference was observed between CME and CRH techniques in postoperative complications while a significant negative median was reported for intraoperative bleeding in favor of CME group. Furthermore, 76.4% of patients undergoing CME surgery were alive at 5 years, compared with 68.2% of CRH.
In our systematic review the results include low rate of postoperative complications analyzed (overall complications 1.88%, anastomotic leak 0.92%, prolonged postoperative ileus 4.71% and reoperation rate 1.51%) and a significant long-term oncological impact of CME and D3 lymphadenectomy surgery (OS 93.05% stage I, 89.76% stage II, 79.65% stage III; TR 5.8% stage I, 7.68% stage II, 15.69% stage III).
Prolonged postoperative ileus is the only complication in evidence, although at a low percentage (4.71%). This functional and non-mechanical inhibition of coordinated gastrointestinal activity may present with nausea or vomiting, inability to tolerate oral food intake, abdominal distension as well as delayed passage of flatus and stool, prolonging hospital stay. Ileus remains one of the commonest complications after elective colorectal surgery, with an estimated incidence of 10–20% following elective colonic resection [52, 53].
Overall, results of our systematic review show an encouraging oncological impact of CME for both survival and recurrence rate, even in stage III cancer.
There are studies included with unbelievable oncological results and postoperative outcomes. One study [20] surprisingly reports 100% OS in any stage of cancer. In this results published by Min Sung An et al. [20], the 5-year OS rate was statistically significantly different: 100% in the CME group versus 89.49% in the non-CME group (p = 0.049), including all tumor stages (UICC stage I, II and III).
Two studies reported no recurrence cases (TR) in stage I, II and III [13, 15]. In the paper by Daxing Xie et al. [13], all the patients received adjuvant chemotherapy, with no case of recurrence. Garcia-Granero et al. [15] showed local recurrence rate of 0% and none of the patients had distal metastasis.
In addiction, six studies in our systematic review report 0% of anastomotic leak, 0% of prolonged postoperative ileus, 0% of gastroplegia and finally 0% of reoperation rate after CME [18, 20, 26, 32,33,34]. Therefore, these relevant data demonstrated a low incidence of complications following CME procedure. Jan Schulte am Esch et al. [18], experienced all Clavien-Dindo grade I-II complications (according to Clavien-Dindo classification scale [54]) and reported no anastomotic leaks, Dindo grade III/IV complications or mortality (Dindo grande V). In the prospective study by W. Petz et al. [26], 18 out of 20 patients had an uneventful postoperative course and 2 patients experienced a Dindo grade IIIa complication. In the prospective study by Benz et al. [32], no relaparoscopy or relaparotomy was required following CME procedure. Also in a retrospective series and then in a prospective RCT, Feng et al. [33, 34] included postoperative complications effectively treated by relevant conservative treatments.
From our revised studies, we also evaluated how MIS is now become the standard for colorectal cancer surgery. However, the learning difficulties of the CME are evident and carries the risk of serious complications, but it would decrease with the standardization of the technique [55]. Postoperative complications did not increase with the age of patients. Consequently, according to the revised studies, elderly patients are not a limit or a contraindication to CME (Table 6). By the way, proposals for prospective multicentre studies would be needed, to evaluate if right colectomy with CME is a necessary and standardized technique in all patients, including early cancer stages (stage I and II) and elderly patients with significant comorbidity.
It should be noted, however, that the definition of CME remains a matter of some debate and the still not-standardization of CME could influence the comparative evaluation of the outcomes from different papers; this may be a limitation of our extensive systematic review. There are no publications on the number of patients needed in the learning curve for CME and for this reason, outcomes may be variable when comparing high and low volume centers. Otherwise, the results would already be much clearer.
Although most are retrospective studies, we also involving prospective observational studies and RCTs that attribute higher quality data to our review. Currently, two prospective randomized studies, RELARC and COLD, are ongoing [56, 57].
In summary, CME is generally thought to be more technically difficult compared to conventional colectomy and necessarily requires a learning curve in colorectal oncologic surgery. Overcome this learning curve, CME does not increase the risk of postoperative complications and significantly improves the long-term oncological impact. However, oncological outcome can only be achieved if optimal surgery is performed. Although outcomes of CME seem to be better than CRH, they should be validated mainly in cohorts of patients with more advanced stage, because in the early stages it may not be justified. Prospective multicentre studies results are needed to verify if CME could be considered standard surgery for right colon cancer, representing an enormous challenge for the current status of colorectal oncologic surgery.
References
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery–the clue to pelvic recurrence? Br J Surg 69(10):613–616
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis 11(4):354–364
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K (2020) Japanese society for cancer of the colon and rectum. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25(1):1–42
West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P (2012) Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 30(15):1763–1769
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 28(2):272–278
Bertelsen CA, Bols B, Ingeholm P, Jansen JE, Neuenschwander AU, Vilandt J (2011) Can the quality of colonic surgery be improved by standardization of surgical technique with complete mesocolic excision? Colorectal Dis 13(10):1123–1129
Kobayashi H, West NP, Takahashi K, Perrakis A, Weber K, Hohenberger W, Quirke P, Sugihara K (2014) Quality of surgery for stage III colon cancer: comparison between England, Germany, and Japan. Ann. Surg. Oncol. 21(3):S398–S404
Bertelsen CA, Neuenschwander AU, Jansen JE, Kirkegaard-Klitbo A, Tenma JR, Wilhelmsen M, Rasmussen LA, Jepsen LV, Kristensen B, Gogenur I, Copenhagen Complete Mesocolic Excision Study, Danish Colorectal Cancer Group (2016) Short-term outcomes after complete mesocolic excision compared with ‘conventional’ colonic cancer surgery. Br J Surg 103:581–589
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Prefferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann intern Med 151:264–269
Benz S (2013) Survival after complete mesocolic excision (CME) for right sided colon cancer compared to standard surgery. Open Surg J 7:6–10
Bertelsen CA, Neuenschwander AU, Jansen JE, Tenma JR, Wilhelmsen M, Kirkegaard-Klitbo A, Iversen ER, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Born PW, Kristensen B, Kleif J (2019) 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol 20(11):1556–1565
Siani LM, Pulica C (2015) Laparoscopic complete mesocolic excision with central vascular ligation in right colon cancer: long-term oncologic outcome between mesocolic and non-mesocolic planes of surgery. Scand J Surg 104(4):219–226
Xie D, Yu C, Gao C, Osaiweran H, Hu J, Gong J (2017) An optimal approach for laparoscopic D3 lymphadenectomy plus complete mesocolic excision (D3+CME) for right-sided colon cancer. Ann Surg Oncol 24(5):1312–1313
Spinoglio G, Bianchi PP, Marano A, Priora F, Lenti LM, Ravazzoni F, Petz W, Borin S, Ribero D, Formisano G, Bertani E (2018) robotic versus laparoscopic right colectomy with complete mesocolic excision for the treatment of colon cancer: perioperative outcomes and 5-year survival in a consecutive series of 202 patients. Ann Surg Oncol 25(12):3580–3586
Garcia-Granero A, Pellino G, Frasson M, Fletcher-Sanfeliu D, Bonilla F, Sánchez-Guillén L, Domenech Dolz A, Primo Romaguera V, Sabater Ortí L, Martinez-Soriano F, Garcia-Granero E, Valverde-Navarro AA (2019) The fusion fascia of Fredet: an important embryological landmark for complete mesocolic excision and D3-lymphadenectomy in right colon cancer. Surg Endosc 33(11):3842–3850
Adamina M, Manwaring ML, Park KJ, Delaney CP (2012) Laparoscopic complete mesocolic excision for right colon cancer. Surg Endosc 26(10):2976–2980
Shin JK, Kim HC, Lee WY, Yun SH, Cho YB, Huh JW, Park YA, Chun HK (2018) Laparoscopic modified mesocolic excision with central vascular ligation in right-sided colon cancer shows better short- and long-term outcomes compared with the open approach in propensity score analysis. Surg Endosc 32(6):2721–2731
Schulte Am Esch J, Iosivan SI, Steinfurth F, Mahdi A, Förster C, Wilkens L, Nasser A, Sarikaya H, Benhidjeb T, Krüger M (2019) A standardized suprapubic bottom-to-up approach in robotic right colectomy: technical and oncological advances for complete mesocolic excision (CME). BMC Surg 19(1):72
Wang Y, Zhang C, Zhang D, Fu Z, Sun Y (2017) Clinical outcome of laparoscopic complete mesocolic excision in the treatment of right colon cancer. World J Surg Oncol 15(1):174
An MS, Baik H, Oh SH, Park YH, Seo SH, Kim KH, Hong KH, Bae KB (2018) Oncological outcomes of complete versus conventional mesocolic excision in laparoscopic right hemicolectomy. ANZ J Surg 88(10):E698–E702
Bae SU, Yang SY, Min BS (2019) Totally robotic modified complete mesocolic excision and central vascular ligation for right-sided colon cancer: technical feasibility and mid-term oncologic outcomes. Int J Colorectal Dis 34(3):471–479
Sheng QS, Pan Z, Chai J, Cheng XB, Liu FL, Wang JH, Chen WB, Lin JJ (2017) Complete mesocolic excision in right hemicolectomy: comparison between hand-assisted laparoscopic and open approaches. Ann Surg Treat Res 92(2):90–96
Ouyang M, Luo Z, Wu J, Zhang W, Tang S, Lu Y, Hu W, Yao X (2019) Comparison of outcomes of complete mesocolic excision with conventional radical resection performed by laparoscopic approach for right colon cancer. Cancer Manag Res 11:8647–8656
Deng X, Hu T, Wei M, Wu Q, Yang T, Meng W, Wang Z (2018) Feasibility of a unidirectionally progressive, pancreas-oriented procedure for laparoscopic D3 right hemicolectomy. Langenbecks Arch Surg 403(6):761–768
Elias AW, Merchea A, Moncrief S, Wise KB, Colibaseanu DT, Dozois EJ, Mathis KL (2020) Recurrence and long-term survival following segmental colectomy for right-sided colon cancer in 813 patients: a single-institution study. J Gastrointest Surg 24(7):1648–1654
Petz W, Ribero D, Bertani E, Borin S, Formisano G, Esposito S, Spinoglio G, Bianchi PP (2017) Suprapubic approach for robotic complete mesocolic excision in right colectomy: oncologic safety and short-term outcomes of an original technique. Eur J Surg Oncol 43(11):2060–2066
He Z, Zhang S, Xue P, Yan X, Zhou L, Li J, Wang M, Lu A, Ma J, Zang L, Hong H, Dong F, Su H, Sun J, Zhang L, Zheng M, Feng B (2019) Completely medial access by page-turning approach for laparoscopic right hemi-colectomy: 6-year-experience in single center. Surg Endosc 33(3):959–965
Spinoglio G, Marano A, Bianchi PP, Priora F, Lenti LM, Ravazzoni F, Formisano G (2016) Robotic right colectomy with modified complete mesocolic excision: long-term oncologic outcomes. Ann Surg Oncol 23(Suppl 5):684–691
Siani LM, Lucchi A, Berti P, Garulli G (2017) Laparoscopic complete mesocolic excision with central vascular ligation in 600 right total mesocolectomies: safety, prognostic factors and oncologic outcome. Am J Surg 214(2):222–227
Kanemitsu Y, Komori K, Kimura K, Kato T (2013) D3 lymph node dissection in right hemicolectomy with a no-touch isolation technique in patients with colon cancer. Dis Colon Rectum 56(7):815–824
Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2014) Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol 21(7):2288–2294
Benz S, Tam Y, Tannapfel A, Stricker I (2016) The uncinate process first approach: a novel technique for laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 30(5):1930–1937
Feng B, Sun J, Ling TL, Lu AG, Wang ML, Chen XY, Ma JJ, Li JW, Zang L, Han DP, Zheng MH (2012) Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: feasibility and technical strategies. Surg Endosc 26(12):3669–3675
Feng B, Ling TL, Lu AG, Wang ML, Ma JJ, Li JW, Zang L, Sun J, Zheng MH (2014) Completely medial versus hybrid medial approach for laparoscopic complete mesocolic excision in right hemicolon cancer. Surg Endosc 28(2):477–483
Kang J, Kim IK, Kang SI, Sohn SK, Lee KY (2014) Laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 28(9):2747–2751
Lee SD, Lim SB (2009) D3 lymphadenectomy using a medial to lateral approach for curable right-sided colon cancer. Int J Colorectal Dis 24(3):295–300
Liang JT, Lai HS, Huang J, Sun CT (2015) Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg Endosc 29(8):2394–2401
Subbiah R, Bansal S, Jain M, Ramakrishnan P, Palanisamy S, Palanivelu PR, Chinusamy P (2016) Initial retrocolic endoscopic tunnel approach (IRETA) for complete mesocolic excision (CME) with central vascular ligation (CVL) for right colonic cancers: technique and pathological radicality. Int J Colorectal Dis 31(2):227–233
Trastulli S, Coratti A, Guarino S, Piagnerelli R, Annecchiarico M, Coratti F, Di Marino M, Ricci F, Desiderio J, Cirocchi R, Parisi A (2015) Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: a retrospective multicentre study. Surg Endosc 29(6):1512–1521
Paquette IM, Madoff RD, Sigurdson ER, Chang GJ (2018) Impact of Proximal Vascular Ligation on Survival of Patients with Colon Cancer. Ann Surg Oncol 25(1):38–45
Toyota S, Ohta H, Anazawa S (1995) Rationale for extent of lymph node dissection for right colon cancer. Dis Colon Rectum 38:705–711
Hohenberger W, Merkel S, Weber K (2007) Lymphadenektomie bei Tumoren des unteren Gastrointestinaltrakts. Chirurg 78:217–225
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Alhassan N, Yang M, Wong-Chong N, Liberman AS, Charlebois P, Stein B, Fried GM, Lee L (2019) Comparison between conventional colectomy and complete mesocolic excision for colon cancer: a systematic review and pooled analysis: a review of CME versus conventional colectomies. Surg Endosc 33(1):8–18
Kim NK, Kim YW, Han YD, Cho MS, Hur H, Min BS, Lee KY (2016) Complete mesocolic excision and central vascular ligation for colon cancer: Principle, anatomy, surgical technique, and outcomes. Surg Oncol 25(3):252–262
Croner RS, Ptok H, Merkel S, Hohenberger W (2018) Implementing complete mesocolic excision for colon cancer—mission completed. Innov Surg Sci 3(1):17–29
Wang C, Gao Z, Shen K, Shen Z, Jiang K, Liang B, Yin M, Yang X, Wang S, Ye Y (2017) Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis 19(11):962–972. https://doi.org/10.1111/codi.13900
Ow ZGW, Sim W, Nistala KRY, Ng CH, Koh FH, Wong NW, Foo FJ, Tan KK, Chong CS (2020) Comparing complete mesocolic excision versus conventional colectomy for colon cancer: A systematic review and meta-analysis. Eur J Surg Oncol 12:S0748-S7983. https://doi.org/10.1016/j.ejso.2020.09.007
Kong JC, Prabhakaran S, Choy KT, Larach JT, Heriot A, Warrier SK (2021) Oncological reasons for performing a complete mesocolic excision: a systematic review and meta-analysis. ANZ J Surg 91(1–2):124–131. https://doi.org/10.1111/ans.16518
Dai Q, Tu S, Dong Q, Chen B (2020) Laparoscopic complete mesocolic excision versus noncomplete mesocolic excision: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech 31(1):96–103. https://doi.org/10.1097/SLE.0000000000000845
De Simoni O, Barina A, Sommariva A, Tonello M, Gruppo M, Mattara G, Toniato A, Pilati P, Franzato B (2020) Complete mesocolic excision versus conventional hemicolectomy in patients with right colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 36(5):881–892
Scarborough JE, Schumacher J, Kent KC, Heise CP, Greenberg CC (2017) Associations of specific postoperative complications with outcomes after elective colon resection: a procedure-targeted approach toward surgical quality improvement. JAMA Surg 152(2):e164681. https://doi.org/10.1001/jamasurg.2016.4681
Chapman SJ, EuroSurg Collaborative (2018) Ileus management international (IMAGINE): protocol for a multicentre, observational study of ileus after colorectal surgery. Colorectal Dis 20(1):O17–O25. https://doi.org/10.1111/codi.13976
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Strey CW, Wullstein C, Adamina M, Agha A, Aselmann H, Becker T, Grützmann R, Kneist W, Maak M, Mann B, Moesta KT, Runkel N, Schafmayer C, Türler A, Wedel T, Benz S (2018) Laparoscopic right hemicolectomy with CME: standardization using the “critical view” concept. Surg Endosc 32(12):5021–5030
Lu JY, Xu L, Xue HD, Zhou WX, Xu T, Qiu HZ, Wu B, Lin GL, Xiao Y (2016) The radical extent of lymphadenectomy—D2 dissection versus complete mesocolic excision of laparoscopic right colectomy for right-sided colon cancer (RELARC) trial: study protocol for a randomized controlled trial. Trials 17:582
Karachun A, Petrov A, Panaiotti L, Voschinin Y, Ovchinnikova T (2019) Protocol for a multicentre randomized clinical trial comparing oncological outcomes of D2 versus D3 lymph node dissection in colonic cancer (COLD trial). BJS Open 3(3):288–298
Acknowledgments
Neither funds nor grants were received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Gennaro Mazzarella, Edoardo Maria Muttillo, Biagio Picardi, Stefano Rossi and Irnerio Angelo Muttillo have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mazzarella, G., Muttillo, E.M., Picardi, B. et al. Complete mesocolic excision and D3 lymphadenectomy with central vascular ligation in right-sided colon cancer: a systematic review of postoperative outcomes, tumor recurrence and overall survival. Surg Endosc 35, 4945–4955 (2021). https://doi.org/10.1007/s00464-021-08529-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08529-4