Abstract
Background
The optimal surgical approach for clinical T4 (cT4) rectal cancer is unknown. This study was conducted to clarify short- and long-term outcomes of robotic surgery for cT4 rectal cancer.
Methods
In our retrospective cohort study, we enrolled patients who underwent robotic surgery for cT4 rectal cancer within 15 cm from the anal verge between 2011 and 2018. The short- and long-term outcomes were evaluated.
Results
Of a total of 122 eligible patients, 70 (57%) had cT4a tumors and 52 (43%) had cT4b tumors. Thirty-five patients (29%) had distant metastasis and 21 (17%) underwent preoperative chemoradiotherapy. Thirty-four patients (28%) underwent combined resection of adjacent organs and 43 (35%) underwent lateral lymph node dissection. The median operative time was 288 min and the median blood loss was 11 ml. No patients required conversion to open surgery. The incidences of postoperative complications of grades II, III, and IV or more according to the Clavien–Dindo classification were 17.2%, 3.5%, and 0%, respectively. Seventy-three patients (60%) had pathological T4 tumors, and the incidence of positive resection margins was 4.9%. The median follow-up time was 42.9 months. The 3-year overall survival, disease-free survival, and cumulative local recurrence rates were 87.5%, 70.4%, and 4.0%, respectively.
Conclusions
The short- and long-term outcomes of robotic surgery for cT4 rectal cancer were favorable. Robotic surgery is considered to be a useful approach for cT4 rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In rectal cancer, T4 tumors are defined as those that directly invade other organs or structures, or perforate the visceral peritoneum [1]. Clinical T4 (cT4) rectal cancer was categorized as having the highest risk of recurrence by both the National Comprehensive Cancer Network Clinical Practice Guidelines and in the European Society for Medical Oncology Clinical Practice Guidelines [2, 3]. Although recent technological developments have provided multiple surgical approaches for rectal cancer, including open surgery, conventional laparoscopic surgery, robotic surgery, and transanal total mesorectal excision [4], the optimal surgical approach is still unknown, especially for cT4 rectal cancer. This is partially because cT4 rectal cancer was excluded in several large, randomized controlled trials: the ROLARR trial comparing robotic surgery and laparoscopic surgery [5], the COREAN trial, the COLOR II trial, the ALaCaRT trial, and the ACOSOG Z6051 trial comparing laparoscopic surgery and open surgery [6,7,8,9]. In robotic surgery for T4 rectal cancer, only one retrospective study evaluated short-term outcomes [10], and no studies have evaluated long-term outcomes. This study was conducted to clarify short- and long-term outcomes of robotic surgery for cT4 rectal cancer.
Materials and methods
Patient selection
This study enrolled patients who underwent robotic surgery for primary rectal adenocarcinoma of cT4 within 15 cm from the anal verge, and all procedures were performed at Shizuoka Cancer Center in Japan between December 2011 and December 2018. The diagnosis of cT4 was based on pretreatment magnetic resonance imaging and all cases were reviewed in a multidisciplinary team conference. The exclusion criteria were double cancer and either synchronous or metachronous colorectal cancer. Written informed consents for examination and treatment were obtained from all patients prior to the procedures. Data collection and analysis were approved by the institutional review board of Shizuoka Cancer Center Hospital (Institutional Code: J2019-162–2019-1–3). Patient characteristics were recorded in a prospective database; these comprised the American Society of Anesthesiologists (ASA) score, body mass index (BMI), presence of preoperative chemoradiotherapy (CRT) and postoperative adjuvant chemotherapy, and the following surgical and pathological details: distance between the lower edge of the tumor and anal verge, clinical and pathological T stage or N stage according to the tumor node metastasis (TNM) classification [1], operative procedure (anterior resection, abdominoperineal resection [APR], or intersphincteric resection [ISR]), and the presence of combined resection of adjacent organs, including the bladder, seminal vesicles, prostate, uterus, ovary, vagina, or pelvic autonomic nerves. Lateral lymph nodes were considered to be regional lymph nodes, as reported previously [11].
In Japan, preoperative CRT is not the standard treatment for locally advanced rectal cancer [12]. Indications for preoperative CRT differ by institution. At our institution, they are limited to avoid its toxicity. Even in patients with rectal cancer that is invading adjacent organs, in this study, we performed surgery without preoperative CRT if it was expected that it would be possible to obtain a clear resection margin (R0) by mesorectal excision with combined resection of the invaded organs. Preoperative CRT was performed only in patients for whom it was predicted that obtaining R0 without CRT would be difficult or for whom shrinkage of the tumor by CRT would make anal preservation possible or permit avoidance of urinary diversion. The external radiotherapy dose was 45 Gy, administered in 25 fractions to a large pelvic field over the course of 5 weeks, plus a boost of 5.4 Gy in three daily fractions, using a four-field approach. Concomitant chemotherapy with the 5-fluorouracil derivative capecitabine (825 mg/m2) was administered orally twice per day, 5 days per week. Operations were performed at 6–10 weeks after CRT [13]. Similarly, in patients who had rectal cancer with distant metastasis, preoperative CRT or preoperative chemotherapy was not performed when it was expected that it would be possible to perform complete resection for primary and metastatic tumors without any preoperative therapy.
ISR was performed when the rectum could not be divided using linear staplers in the abdominal approach. APR was performed if the tumor invaded the levator ani muscle or was of the macroscopic infiltrating type or if fecal continence was impaired. Lateral lymph node dissection was performed when the lower border of the tumor was located distal to the peritoneal reflection [12].
Robotic surgery was introduced in December 2011 at our institution. In principle, the indication for robotic surgery was rectal adenocarcinoma of clinical stage 0–IV. Robotic surgery for rectal cancer was not covered by national public health insurance in Japan until March 2018. Therefore, it was a costlier treatment option than laparoscopic or open surgery. After patients provided informed consent, they indicated their preference for robotic, laparoscopic, or open surgery, and the procedure was selected accordingly. All treatment strategies, including operative approaches or procedures, were approved in a multidisciplinary team conference at our institution.
In patients with pathological stage III or patients with pathological stage IV who underwent complete resection for primary and metastatic tumors, 5-fluorouracil-based adjuvant chemotherapy was administered to patients under 75 years old who did not have any severe comorbidity.
Operative technique
All procedures were performed robotically using a systematic approach that included a colonic and pelvic phase [14]. During the colonic phase, high ligation of the inferior mesenteric artery was performed via the medial-to-lateral approach. The pelvic phase involved rectal mobilization keeping mesorectal plane by sharp dissection. If tumor invasion beyond the mesorectum was suspected, en bloc resection of adjacent organs or pelvic autonomic nerves was performed [15].
Each trocar was placed as shown in Fig. 1. Figure 2 shows intraoperative pictures in robotic low anterior resection plus total hysterectomy and partial vaginectomy for rectal cancer invading vagina. Rectum, uterus, and vagina were mobilized en block (Fig. 2a). After incision of anterior vaginal wall, posterior and lateral vaginal walls and parametrium were resected (Fig. 2b). Rectum was divided by using linear staplers (Fig. 2c). Colorectal anastomosis was performed by using double stapling technique after closure of vaginal stump (Fig. 2d). Figure 3 shows an intraoperative picture in robotic low anterior resection plus resection of seminal vesicles for rectal cancer invading seminal vesicles.
Low anterior resection plus total hysterectomy and partial vaginectomy for rectal cancer invading vagina. A Mobilization of rectum, uterus, and vagina. B Resection of left vaginal wall and parametrium. C Division of rectum by using linear staplers. D Colorectal anastomosis by using double stapling technique after closure of vaginal stump
Surveillance protocol
Surveillance was performed for 5 years after surgery. The surveillance protocol at our institution consisted interviews, physical examinations, and blood tests, including carcinoembryonic antigen and CA-19–9 antigen, every 3 months for the first 3 years after surgery and then every 6 months thereafter. Chest, abdominal, and pelvic computed tomography was performed every 6 months. Colonoscopy was performed annually for the first 3 years after surgery. Recurrence was confirmed pathologically or by progressively increasing tumor size in imaging studies.
Outcome measurements
The primary short-term outcome in this study was the incidence of 30-day postoperative complications after surgery, classified according to the Clavien–Dindo classification [16]. Urinary retention was defined as the presence of more than 50 ml residual urine volume of the measured value after voiding. As for other surgical outcomes, intraoperative blood loss, operative time, the incidence of conversion to open surgery, and postoperative hospital stay were collected from a prospective database. The number of harvested lymph nodes and the incidence of positive resection margins were also evaluated. A positive resection margin included a positive surgical dissection plane and a positive proximal or distal margin of the resected specimen [17]. As long-term outcomes, overall survival (OS), disease-free survival (DFS), and local recurrence (LR) rates were determined. DFS was evaluated in patients without distant metastasis.
Statistical analysis
Categorical variables are described as numbers and percentages, and continuous variables are presented as medians (range). OS and DFS rates were calculated using the Kaplan–Meier method. The cumulative incidence was used to estimate LR rates with death as a competing risk. All statistical analyses were performed using JMP software, version 13.0 (SAS Institute, Cary, NC, USA) and the statistical program R version 3.0.2 (http://www.r-project.org/).
Results
Patient characteristics
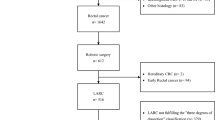
A total of 126 patients underwent robotic surgery for cT4 rectal cancer between 2011 and 2018. Patients who had multiple colorectal cancers (n = 4) were excluded. The remaining 122 patients were analyzed. Table 1 provides an overview of the patient characteristics. The median age was 64 years (range, 31–86 years), and 77 patients (63.1%) were male. The median distance from the tumor to the anal verge was 7.0 cm (range, 0–15.0 cm). Seventy patients (57.4%) had cT4a tumors and 52 patients (42.6%) had cT4b tumors. Thirty-five patients (28.7%) had distant metastasis. Twenty-one patients (17.2%) underwent preoperative CRT. In patients with distant metastasis, two patients underwent preoperative CRT and one underwent chemotherapy prior to surgery. Fifty-nine patients underwent postoperative adjuvant chemotherapy. In 52 patients with pathological stage III rectal cancer, 38 patients (73.1%) underwent adjuvant chemotherapy. In 35 patients with distant metastasis, 28 patients underwent complete resection for primary and metastatic tumors, and 17 patients underwent adjuvant chemotherapy.
Surgical and pathological outcomes
Perioperative outcomes are presented in Table 2. Ninety-four patients (77.0%) underwent sphincter-preserving surgery, 34 (27.9%) underwent combined resection of adjacent organs, 21 (17.2%) underwent combined resection of the pelvic autonomic nerves, and 43 (35.2%) underwent lateral lymph node dissection. In 70 patients with cT4a rectal cancer, three patients required combined resection of adjacent organs. In 52 patients with cT4b rectal cancer, 31 patients underwent combined resection of adjacent organs, 14 patients underwent only APR, in which levator ani muscle was resected, and seven patients did not require combined resection of adjacent organs based on intraoperative findings. No patients required conversion to open surgery. The median operative time was 288 min (range, 149–574 min), the median blood loss was 11 ml (range, 0–502 ml), and no patients received transfusions. In patients without lateral lymph node dissection, the median operative time was 217 min (range, 149–520 min) and the median blood loss was 5 ml (range, 0–502 ml). Postoperative outcomes are presented in Table 3. The incidences of postoperative complications of grade II, III, and IV or more according to the Clavien–Dindo classification were 17.2%, 2.5%, and 0%, respectively. The incidence of anastomotic leakage was 2.5%. The median postoperative hospital stay was 7 days. No patients died perioperatively. Pathological outcomes are presented in Table 4. Seventy-three patients (59.8%) had pT4 tumors, 19 (15.6%) had tumors demonstrating adjacent organ invasion on pathological examination, and 85 (69.7%) had lymph node metastasis. The median number of harvested lymph nodes was 41 (range, 16–87). The incidence of positive resection margins was 4.9%.
Long-term outcomes
The median follow-up time was 42.9 months. Figures 4, 5, and 6 show the OS, RFS, and cumulative LR curves, respectively. OS and cumulative LR were evaluated in all patients (n = 122), and DFS was evaluated in patients without distant metastasis (n = 87). The 3-year OS, DFS, and cumulative LR rates were 87.5%, 70.4%, and 4.0%, respectively. Local recurrence occurred in the central pelvis in two patients, in the lateral pelvis in one patient, and in the anastomosis in one patient.
Discussion
This is the first report of both short- and long-term outcomes of robotic surgery for cT4 rectal cancer. The outcomes were favorable for both time frames. Regarding short-term outcomes, the incidences of postoperative complications of grades II, III, and IV or more according to the Clavien–Dindo classification were 17.2%, 3.5%, and 0%, respectively. No patients required conversion to open surgery. The incidence of positive resection margins was 4.9%. Crolla et al. evaluated short-term outcomes in 28 patients undergoing robotic surgery for cT4b rectal and distal sigmoid cancer [10]. They reported that the incidence of postoperative complications of grade III or more was 14%, the incidence of conversion to open surgery was 11%, and the incidence of R1 resection was 14%. Our outcomes are therefore superior, even though both patients with cT4a and cT4b rectal cancer were enrolled in this study. In laparoscopic and open surgery, de’Angelis et al. evaluated short-term outcomes in patients undergoing surgery for pT4 rectal cancer, and showed that patients with pT4b rectal cancer comprised 21% and 33% of those who underwent laparoscopic and open surgery, respectively [18]. They reported that the incidences of postoperative complications of grade III or more were 21% and 27% in laparoscopic and open surgery, respectively, the incidence of conversion to open surgery was 21% in laparoscopic surgery, and the incidences of positive circumferential resection margins were 15% and 14% in laparoscopic and open surgery, respectively. Another study evaluating the short- and long-term outcomes of laparoscopic surgery for cT4 colorectal cancer reported that in rectal cancer specifically, the incidence of conversion to open surgery was 16% and the incidence of positive resection margins was 17% [19]. For T4 rectal cancer, the short-term outcomes of robotic surgery were considered to be more favorable than those of laparoscopic or open surgery. As for long-term outcomes, the 3-year OS and cumulative LR rates in all patients were 87.5% and 4.0%, respectively, and the 3-year DFS rate in patients without distant metastasis was 70.4%. No studies thus far have evaluated the long-term outcomes of robotic surgery for T4 rectal cancer. In laparoscopic surgery for pT4 rectal cancer, the 3-year OS and DFS rates were reported to range from 66.7 to 79.1% and from 55.4 to 68.6%, respectively [18, 20]. In open surgery for pT4 rectal cancer, the 3-year OS and DFS rates were reported to range from 64.1 to 71.8% and from 53.3 to 66.7%, respectively [18, 20]. In several large randomized controlled trials comparing laparoscopic to open surgery for cT1–T3 rectal cancer, LR rates at 2 or 3 years after surgery were reported to range from 2.6 to 5.0% with laparoscopic surgery and from 3.1 to 5.0% with open surgery [21,22,23,24], which were comparable to the finding in this study of a 3-year cumulative LR rate of 4.0% with robotic surgery for cT4 rectal cancer. Therefore, robotic surgery for cT4 rectal cancer may have a better long-term outcome than laparoscopic or open surgery. Multivisceral resection is often required in cT4 rectal cancer surgery, as demonstrated by the fact that about 30% of patients in our study underwent combined resection of adjacent organs. Several previous studies reported the usefulness of robotic surgery for rectal cancer requiring multivisceral resection [10, 15, 25]. Shin et al. reported that the 5-year OS, DFS, and LR rates were 80.0%, 54.6%, and 3.6%, respectively, in 32 patients undergoing robotic multivisceral resection, lateral lymph node dissection, or retroperitoneal lymph node dissection, including ten patients (27.8%) with cT4 rectal cancer [25]. Thus, the distinctive benefits of robotic surgery, which include a stable, three-dimensional view of the surgical field, multi-articulated instruments, digital suppression of physiologic hand tremor, and motion scaling, are considered to apply even in T4 rectal cancer, and together they enable surgeons to perform precise, sharp dissection while maintaining proper surgical planes, even in patients with a deep, narrow pelvis [26].
According to Japanese Society for Cancer of the Colon and Rectum guidelines for the treatment of colorectal cancer [12], adjuvant chemotherapy was basically recommended for patients with pathological stage III cancer and patients with distant metastasis who underwent complete resection for primary and metastatic tumors. In our study, the proportion of patients who underwent adjuvant chemotherapy in patients with stage III and in stage IV rectal cancer after complete resection was 73.1% (38 of 52) and 60.7% (17 of 28), respectively. In pathological stage III rectal cancer, a Japanese randomized controlled trial demonstrated adjuvant chemotherapy had significant advantage over surgery alone in terms of both OS and DFS [27]. Further analyses were needed to evaluate adjuvant chemotherapy for patients with distant metastasis who underwent complete resection for primary and metastatic tumors.
In this study, patients who had cT4 rectal cancer within 15 cm from the anal verge were enrolled because they were excluded from the ROLARR randomized clinical trial comparing robotic surgery with laparoscopic surgery for rectal cancer [5], and the data of clinical outcomes of robotic surgery for these patients were lacking. The concordance rate between clinical and pathological T stage was 59.8% in our study, which was not lower than the range of 25.4–41.9% in the previous studies on T4 colorectal cancer [10, 28].
There are several limitations to this study. First, it used a retrospective, single-center design and was not a comparative study of open and laparoscopic surgery. Especially in T4 rectal cancer surgery, it was difficult to compare clinical outcomes following robotic surgery to those following laparoscopic or open surgery because clinical characteristics of patients were much different by each operative approach. Procedures requiring urinary diversion, such as ureteroneocystostomy and ileal conduit, were conducted using open surgery rather than robotic surgery because the latter is not yet used for these indications at our institution. In principal, lateral lymph node dissection was performed for patients with cT4 lower rectal cancer, and that was basically performed by robotic or open surgery, not performed by laparoscopic surgery [29]. Therefore, patients with cT4 lower rectal cancer rarely underwent laparoscopic surgery. Second, we had no data regarding circumferential resection margin, which is not generally evaluated in Japan [30]. Third, the learning curve in robotic surgery for rectal cancer was not taken into account in this study.
In conclusion, we demonstrated favorable short- and long-term outcomes of robotic surgery for cT4 rectal cancer. Robotic surgery is considered to be a useful approach for cT4 rectal cancer.
References
Brierley JD, Gospodarowicz MK, Wittekind C (2017) TNM classification of malignant tumours, 8th edn. Wiley-Blackwell, Oxford
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA (2018) Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16:874–901
Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D, ESMO Guidelines Committee (2017) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv22–iv40
Dayal S, Battersby N, Cecil T (2017) Evolution of surgical treatment for rectal cancer: a review. J Gastrointest Surg 21:1166–1173
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 318:1569–1580
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645
van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ, Colorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J, ALaCaRT Investigators (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314:1356–1363
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 314:1346–1355
Crolla RMPH, Tersteeg JJC, van der Schelling GP, Wijsman JH, Schreinemakers JMJ (2018) Robot-assisted laparoscopic resection of clinical T4b tumours of distal sigmoid and rectum: initial results. Surg Endosc 32:4571–4457
Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K, Japanese Society for Cancer of the Colon and Rectum (2012) Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg 255:1129–1134
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K, Japanese Society for Cancer of the Colon and Rectum (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1–42
Yamaoka Y, Kagawa H, Shiomi A, Yamakawa Y, Hino H, Manabe S, Kinugasa Y (2020) Robotic-assisted surgery may be a useful approach to protect urinary function in the modern era of diverse surgical approaches for rectal cancer. Surg Endosc. https://doi.org/10.1007/s00464-020-07509-4
Shiomi A, Kinugasa Y, Yamaguchi T, Tomioka H, Kagawa H (2014) Robot-assisted rectal cancer surgery: short-term outcomes for 113 consecutive patients. Int J Colorectal Dis 29:1105–1111
Hino H, Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Numata M, Furutani A, Yamaoka Y, Manabe S, Suzuki T, Kato S (2017) Robotic-assisted multivisceral resection for rectal cancer: short-term outcomes at a single center. Tech Coloproctol 21:879–886
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Furutani A, Manabe S, Yamaoka Y, Hino H (2018) Oncological outcomes of robotic-assisted laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer. Surg Endosc 32:4498–4505
de′Angelis N, Landi F, Vitali GC, Memeo R, Martínez-Pérez A, Solis A, Assalino M, Vallribera F, Mercoli HA, Marescaux J, Mutter D, Ris F, Espin E, Brunetti F (2017) Multicentre propensity score-matched analysis of laparoscopic versus open surgery for T4 rectal cancer. Surg Endosc 31:3106–3121
Bretagnol F, Dedieu A, Zappa M, Guedj N, Ferron M, Panis Y (2011) T4 colorectal cancer: is laparoscopic resection contraindicated? Colorectal Dis 13:138–143
Zhang X, Wu Q, Hu T, Gu C, Bi L, Wang Z (2019) Laparoscopic versus conventional open surgery in T4 rectal cancer: a case-control study. J Minim Access Surg 15:37–41
Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim DH, Kim JS, Lee HS, Kim JH, Oh JH (2014) Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 15:767–774
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E, COLOR II Study Group (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372:1324–1332
Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Wilson K, Hague W, Simes J, Australasian Gastro-Intestinal Trials Group (AGITG) ALaCaRT investigators (2019) Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the Australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg 269:596–602
Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR Jr, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H (2019) Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg 269:589–595
Shin US, Nancy You Y, Nguyen AT, Bednarski BK, Messick C, Maru DM, Dean EM, Nguyen ST, Hu CY, Chang GJ (2016) Oncologic outcomes of extended robotic resection for rectal cancer. Ann Surg Oncol 23:2249–2257
Shiomi A, Kinugasa Y, Yamaguchi T, Kagawa H, Yamakawa Y (2016) Robot-assisted versus laparoscopic surgery for lower rectal cancer: the impact of visceral obesity on surgical outcomes. Int J Colorectal Dis 31:1701–1710
Sakamoto J, Hamada C, Yoshida S, Kodaira S, Yasutomi M, Kato T, Oba K, Nakazato H, Saji S, Ohashi Y (2007) An individual patient data meta-analysis of adjuvant therapy with uracil-tegafur (UFT) in patients with curatively resected rectal cancer. Br J Cancer 96:1170–1177
Park JS, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, Lee WY, Chun HK (2016) Clinically suspected T4 colorectal cancer may be resected using a laparoscopic approach. BMC Cancer 16:714
Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H (2016) Robotic-assisted laparoscopic versus open lateral lymph node dissection for advanced lower rectal cancer. Surg Endosc 30:721–728
Japanese Society for Cancer of the Colon and Rectum (2019) Japanese classification of colorectal, appendiceal, and anal carcinoma, third English edition. Kanehara & CO, Ltd, Tokyo
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Yusuke Yamaoka, Dr. Akio Shiomi, Dr. Hiroyasu Kagawa, Dr. Hino Hitoshi, Dr. Shoichi Manabe, Dr. Shunichiro Kato, and Dr. Marie Hanaoka have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamaoka, Y., Shiomi, A., Kagawa, H. et al. Robotic surgery for clinical T4 rectal cancer: short- and long-term outcomes. Surg Endosc 36, 91–99 (2022). https://doi.org/10.1007/s00464-020-08241-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08241-9