Abstract
Locally advanced rectal cancer often requires an extended resection beyond the total mesorectal excision plane (bTME) to obtain clear resection margins. We classified three types of bTME rectal cancer following local disease diffusion: radial (adjacent pelvic organs), lateral (pelvic lateral lymph nodes) and longitudinal (below 3.5 cm from the anal verge, submitted to intersphincteric resection). The primary aim of this study was to evaluate the application of robotic surgery to the three types of bTME regarding the short and long-term oncological outcomes. Secondary aim was to identify survival prognostic factors for bTME rectal cancers. A total of 137 patients who underwent robotic-assisted bTME procedures between 2008 and 2018 were extracted from a prospectively collected database. Patient-related, operative and pathological factors were assessed. Morbidity was moderately high with 66% of patients reporting postoperative complications. Median follow up was 47 months (IQR, 31.5–66.5). Local recurrence rate was 15.3% with a statistical difference between the three types of bTME (p = 0.041). Disease progression/distant metastasis rate was 33.6%. Overall survival was significantly different (p = 0.023) with 1- and 3-years rates of: 77.8% and 55.0% (radial; n = 19); 96.6% and 84.8% (lateral; n = 30); 97.7% and 86.9% (longitudinal; n = 88). No statistical difference was observed for disease-free survival (p = 0.897). Local recurrence-free survival was significantly different between the groups (p = 0.031). Multivariate analysis showed that (y)pT (p = 0.028; HR (95% CI) 5.133 (1.192–22.097)), (y)pN (p = 0.014; HR (95% CI) 2.835 (1.240–6.482)) and type of bTME were associated to OS whilst (y)pT (p = 0.072) and type of bTME were not associated to LRFS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rectal surgery has greatly improved in the last decades. Total mesorectal excision (TME) for the treatment of rectal cancer described by Heald in 1982 [1] has become the gold standard for rectal surgery. The key concept of dissection through embryological planes for the removal of rectal cancer together with its draining perirectal tissue (mesorectum) has reduced the local recurrence (LR) rate down to 4% at 5 years after surgery [2]. The application of extensive screening programs, the acknowledgement of the role of neoadjuvant treatments in reducing LR [3], the introduction of minimally invasive approaches and the progress in the use of magnetic resonance imaging (MRI) have been further steps in rectal cancer treatment evolution.
Despite these progress, around 5–10% of patients with locally advanced rectal cancer (LARC) present further involvement of adjacent organs [4]. These patients require an extended surgical resection “beyond the TME plane” to achieve a pathological R0 resection, which is an important prognostic factor for LR and overall survival (OS) for LARC [5,6,7].
To optimize the treatment, these patients must be discussed in a multidisciplinary team (MDT) [4]. The combination of neoadjuvant treatment and extended surgery has increased the 5-year survival for LARC up to 70% with, however, a significant morbidity around 25% [6, 8].
In our center, patients with rectal tumors invading adjacent pelvic organs (radial diffusion) and/or pelvic lateral lymph node involvement (lateral diffusion) and/or very low lying rectal cancers requiring an intersphincteric resection (ISR, distal longitudinal diffusion) are classified as bTME rectal cancers.
Few reports, discussing the role of robotic surgery for bTME rectal tumors, are available in the literature and these often concentrate only on a single district of tumor spreading with no report evaluating the oncological outcomes of bTME cancers as a whole [9,10,11].
The present study aimed primarily to evaluate the short and long-term oncological outcomes of the three type of bTME submitted to robotic surgery. Secondary aim was to identify survival prognostic factors for bTME rectal cancers.
Materials and methods
Study population and database characteristics
A retrospective study evaluating patients with LARC submitted to robotic bTME was performed extracting data from the prospectively maintained colorectal database at the Korea University Anam Hospital, a tertiary referral center in Seoul, South Korea. The study was approved by the Institutional Review Board (#2020AN0364).
In our center, rectal tumors invading adjacent pelvic organs (radial diffusion) and/or pelvic lateral lymph node involvement (lateral diffusion) and/or very low lying rectal cancers requiring an ISR (below 3.5 cm from the anal verge; distal longitudinal diffusion) are classified as bTME rectal cancers because the dissection plane for a radical resection lays outside the boundaries of standard TME. This anatomical classification according to the surgical plane defines our novel “three degrees of dissection” classification of bTME rectal cancers: radial, lateral and distally longitudinal.
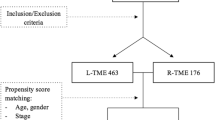
Data were collected from January 2008 to May 2018. A total of 137 patients fulfilled the following inclusion criteria: (1) primary LARC (T stage ≥ 3 or any T with N +); (2) adenocarcinoma in histology; (3) preoperative signs of direct invasion to adjacent pelvic organs (radial group) or lateral pelvic lymph node involvement (lateral group) or low lying rectal cancer (≤ 3.5 cm from the AV, undergoing ISR; longitudinal group); (4) elective surgery; (5) robotic-assisted approach; (6) no previous history of colorectal cancer; (7) no evidence of synchronous colorectal cancer; (8) no history of hereditary cancer syndromes (Fig. 1).
In the study period, tumor invasion above the S2 sacral bone was considered a contraindication for radical surgery because of severe morbidity associated with high sacral resections.
The distance between the tumor and the AV was assessed via digital rectal examination and rectal MRI. Clinical staging was performed via colonoscopy with biopsy, MRI, thoracic and abdomino-pelvic computed tomography (CT). The final pathologic features were restaged according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system [12] at the time of data review.
Every patient was discussed in a MDT comprising: colorectal surgeons, pathologists, radiologists and radiotherapists with liver, lung surgeons, gynecologists and urologists when required. The treatment strategy was neoadjuvant chemo-radiotherapy (CRT; long course, 5080 cGy administered in 28 fractions and 5-fluorouracil based chemotherapy or short course, 2500 cGy administered in 5 fractions) followed by surgery or surgery only (in highly selective cases of feasible R0 resection within a bTME dissection).
The institutional neoadjuvant CRT policy was established according to our previous data [13, 14]. Surgery was performed 8–10 weeks after neo-adjuvant CRT and clinical restaging with pelvic MRI and thoracic/abdomen/pelvic CT.
Postoperative follow-up protocol included physical examination and serum CEA assay every 3 months for the first 2 years thereafter every 6 months. Chest and abdomino-pelvic CTs were taken every 6 months for the first 2 years then annually for the following years. Colonoscopy and sigmoidoscopy were performed alternatively every 6 months for the first 2 years, whilst a colonoscopy was performed annually afterwards. Additional tests, including pelvic MRI or positron emission tomography scans, were performed on an as-needed basis.
Post-operative morbidity and mortality rates have been prospectively updated, on a weekly basis, in the colorectal database since 2012.
Overall survival (OS) was measured from the date of surgery to that of death or last follow-up. Disease-free survival (DFS) was measured from the date of surgery to that of tumor recurrence. Local recurrence-free survival (LRFS) was measured from the date of surgery to that of local recurrence. Recurrence was diagnosed through radiological detection of lesions with increasing size or by histological confirmation. LR were classified according to the Dutch TME trial classification [15].
This study is in accordance with the STROBE statement for cohort studies [16].
Surgical technique
All surgeries were performed with robotic-assisted approach by three surgeons with expertise in robotic colorectal surgery (SHK, JK and JMK). The robotic platform used was da Vinci Si® or Xi® (Intuitive Surgical, Inc., Sunnyvale, CA, USA) according to availability. The robotic approach was performed with a two stage-single docking procedure without changing the position of the patient-side surgical cart and, in the case of ISR, was comprised of five steps [17]. The surgical technique for ISR was previously described [18] together with a detailed analysis of the anatomical landmarks for an optimal identification of the intersphincteric plane [19]. Pelvic lateral lymph node dissection (PLLND) was performed monolaterally or bilaterally according to preoperative images of lymph node involvement. The surgical procedure of robotic-assisted PLLND had been described elsewhere [20]. The surgical extension of the multivisceral resections was decided preoperatively, and not intraoperatively, according to the surgical dissecting planes based on staging MRI images (Fig. 2).
a intraoperative view of a robotic dissection of the left pelvic lateral nodes (lateral bTME) with preservation of the obturator nerve (white arrow); (b) intraoperative view of a robotic exenteration (radial bTME) for an upper rectal cancer invading the bladder and both seminal vesicles with prostate preservation and neo-bladder formation. Preoperative MRI are visible with the reported, preoperatively decided, line of dissection; (c) bladder neck containing the entire prostate (white arrow) is seen after robotic exenteration of the involved pelvic organs; (d) intraoperative view of a robotic intersphincteric resection. Dissection of the intersphincteric plane (white line) on the right side of the pelvis, between the rectum medially and the levator ani muscle (LAM) laterally
Statistical analysis
Patient characteristics were summarized using basic descriptive statistics. Continuous variables were presented as median (interquartile range, IQR) or mean ± standard deviation, accordingly, and compared using the Mann–Whitney U test. Categorical variables were expressed as proportions and analyzed using the Chi-squared or Fisher’s exact test. All statistical analysis was performed using SPSS, version 26.0 (IBM Corporation, Armonk, NY, USA). Univariate Cox proportional hazards regression survival analysis was used to analyze the risk factors for OS and LRFS. Factors with p < 0.10 in univariate analysis were further examined using multivariable Cox proportional hazard regression. The Kaplan–Meier model was used to calculate survival and recurrence rates. Confidence intervals were estimated at 95%, and the significance level was set at p = 0.05.
Results
Preoperative characteristics
A total of 137 patients fulfilling the inclusion criteria were enrolled in the study. Patients and primary tumor characteristics are listed in Table 1. The median age was higher in the lateral and longitudinal bTME group compared to the radial group and the majority of patients were male in all groups. Tumor size and stage were significantly different between the bTME groups (p < 0.001). Also the N and M stages were significantly different (p = 0.005 and p = 0.008, respectively). Distant metastasis occurred in 28 patients (20.4%) including lung (n: 9), liver (n: 5), LN (n: 7), carcinoma peritonei (n: 1) and multiple metastases (n: 6).
Following our institutional neoadjuvant policy, around 90% of patients were submitted to preoperative CRT in the radial and lateral group with only 76.1% in the longitudinal group due to the inferior T and N stage. A total of 17 patients received preoperative CRT (all short course) in the radial group, whilst 27 patients (74.1% short course, 25.9% long course) received it in the lateral group and 67 (7.5% short course, 91.0% long course, 1.5% chemotherapy only) in the longitudinal group.
Surgical characteristics and short-term outcomes
Neither conversion (to laparoscopy or open) nor intraoperative death was observed in the series. A total of three patients (1.6%) underwent a palliative procedure for local control. Median blood loss was higher in the radial group but no statistical difference was observed between the three bTME groups (p = 0.266). Median operating time was longer in the radial group: 347 min (IQR, 301–410). Surgical procedures are reported in Table 1. A total of eight patients of the lateral group were also submitted to ISR for achieving an oncologically safe anal sparing resection. These patients, however, had tumors at a mean distance of 5.4 cm from the AV, therefore, higher than the value considered as an indication criteria for ISR in our center [19]. For this reason, they were included in the lateral group and not in the longitudinal group.
The radial group comprehended: 4 prostatectomies, 2 cystectomies, 1 bladder posterior wall excision, 8 seminal vesicles removal, 4 hysterectomies, 4 posterior vaginal wall excision, 3 oophorectomies, 1 sacrectomy and 2 coccygectomies.
One patient died within 3 months from surgery (0.7%). Post-operative hospital stay was significantly different between the three bTME groups (p = 0.011).
Postoperative complications were reported in 48.9% of patients (n = 67) with ileus being the most common (23.4%) followed by urinary retention (10.8%; 87% of which required Foley catheter reinsertion), chylous ascites (10.2%), anastomotic leakage (9.5%), intraabdominal/pelvic abscess 2.9%, stoma complications (2.2%; one parastomal abscess, one peristomal cellulitis and one acute ileostomy prolapse) and sepsis (0.7%). According to Clavien-Dindo classification [21], 40 patients had a complication grade I–II whilst 27 patients had a grade III–IV. A total of three patients required surgical treatment: one patient with colo-rectal anastomotic leakage underwent laparoscopic abdominal irrigation and drainage; another patient with vesico-urethral anastomosis site leakage needed an open cystostomy; the patient with ileostomy prolapse, occurring on post-operative day one, required surgical revision.
Pathology of resected specimens
Pathological T and N stage are reported in Table 1. A higher rate of cT4 was observed in the radial group (n: 12, 63.3%) compared to the other bTME groups (p < 0.001). Lymph node metastasis was more frequent in the radial and lateral groups compared to the longitudinal group (p = 0.002). In the lateral group, on a median of 5 (range 0–17) retrieved nodes, 0.7 (range 0–3) were positive.
A total of 92.7% patients were reported R0: 89.5% in the radial, 90% in the lateral and 94.3% in the longitudinal group. However, no statistical difference occurred in the resection margin status between the three bTME groups (p = 0.214). All 3 case of R2 were from palliative resections. The R1 cases were consequent to radial involvement in the radial group (n: 1) and longitudinal group (n: 3) and distal involvement in the lateral group (n:1) and longitudinal group (n: 2).
Long term oncological outcomes
At the time of the study, the median follow-up was 47 months (IQR, 31.5–66.5). LR occurred in 21 patients (15.3%) (Table 1). There was a statistical difference in LR rate between the three bTME groups with the lateral group reporting up to 30% (n: 9) (p = 0.041).
Disease progression/distant metastasis occurred in 46 patients (33.6%) including lung (n: 23), liver (n: 10), nodal (n: 3), bone (n: 2) and multiple metastases (n: 8). Ten patients had both local and systemic recurrences. Local recurrence pattern was: anterior (n: 2), presacral (n: 3), lateral (n: 9), axial (n: 6), and perineal (n: 1).
One and three years OS rates were: 77.8% and 55.0% for the radial group; 96.6% and 84.8% for the lateral group; 97.7% and 86.9% for the longitudinal group (Fig. 3a). OS was significantly different between the three bTME groups (p = 0.023).
One and three years’ disease-free survival (DFS) rates were: 65.8% and 59.8% for the radial group; 78.7% and 50.2% for the lateral group; 88.6% and 58.7% for the longitudinal group (Fig. 3b). DFS was not significantly different between the three bTME groups (p = 0.897).
One and three years’ local recurrence-free survival (LRFS) rates were: 87.7% for the radial group (3-years LRFS not available); 85.8% and 68.8% for the lateral group; 96.5% and 87.9% for the longitudinal group (Fig. 3c). LRFS was significantly different between the three bTME groups (p = 0.031). If the radial group had the worst OS, the lateral group reported the worst LRFS.
The result of the univariate Cox regression analyses for OS and LRFS are reported in Table 2. Multivariate analysis showed that (y)pT, (y)pN and type of bTME were associated with OS whilst (y)pT and type of bTME were not quite associated with LRFS. No significant association between local recurrence and resection margin was reported (Table 3).
Discussion
This study shows our experience in robotic-assisted bTME and reports the oncological long term outcomes in an experienced center for minimally invasive colorectal surgery for LARC.
Literature lacks a precise and shared definition of LARC and bTME. Several studies and trials have defined the LARC to standardize the scientific terminology, however, there are some differences between them: EXPERT study (T1–4 N2; low T3; T4; threatened CRM); MERCURY study (T3c, T3d or T4); German CAO/ARO/AIO-94 trial (any T N + , or T3/T4) [22,23,24]. In our institution, we define LARC according to the German trial [24] and we follow the treatment strategy according to the European Society of Medical Oncology (ESMO) guidelines [25]. However, in case of suspicious lateral LN (common, internal and external iliac and obturator) a surgical resection of the lateral lymphatic compartment is performed monolaterally or bilaterally (according to the location) as described by Kim et al. [20].
Moreover, in case of low lying rectal cancer (below 4 cm from the AV) we always propose an ISR as a first option, if feasible, according to our institutional guidelines [19]. Pelvic lateral lymph node dissection (PLLND) and ISR, together with extended rectal resection to adjacent pelvic organs are considered as bTME surgical treatments because the dissection plane lays outside the boundaries of standard TME. We classify the bTME dissection anatomically according to the surgical plane into radial (adjacent pelvic organs), lateral (pelvic lymph node involvement) and distally longitudinal (ISR). This classification is novel according to our knowledge and goes further on from previous definitions of bTME by other authors [7, 26,27,28]. In 2013 the Beyond TME Collaborative has published a consensus statement on the multidisciplinary management of patients with bTME primary and recurrent cancer [4]. They defined a bTME primary rectal cancer as a tumor which is “predicted by MRI to require an extended surgical resection beyond the TME plane to achieve a pathological R0 resection”. Our definition of “three degrees of dissection” (radial, lateral and longitudinal) is compliant to this previous definition and also aims to standardize the surgical treatment for bTME to evaluate the patients as a “whole” and not according to a “surgical treatment classification”.
To have a high-quality strategy for bTME rectal cancer an experienced MDT is of paramount importance for achieving a R0 resection. Cross-disciplinary skills and multispecialty collaboration with experience in managing complex cases are required to provide a coordinated team approach [29]. Moreover, state-of-the-art diagnostic, therapeutic and interventional radiology, advanced intensive care facilities and an available blood-bank support are needed for optimal treatment strategy [29].
Radical surgery for bTME involves precise knowledge of the pelvic anatomy for both the deep pelvis during ISR, the lymphatic lateral compartment and the anterior genito-urinary district with the precious assistance from other surgical specialties. The present series confirms that bTME resections can be performed with a high R0 resection rate, low mortality and with good oncological long-term outcomes also through the use of the robotic platform. We report similar morbidity and mortality of previous studies performed with open approach [7, 26,27,28].
In the present study, there are clear differences between the three type of bTME regarding pathological (the TNM stage (both clinical and pathological), the tumor size, the distance from the AV, the preoperative CA19.9 blood levels), surgical (the DRM extension, the duration of surgery, the post-operative stay) and prognostic characteristics (the OS and LR rate). The differences of the oncological variables are consequent to the differences between the three types of bTME rectal tumors with worst T stage and tumor size occurring in the radial group and worst N stage in the lateral group. Performing both PLLND and ISR are challenging and time consuming even for experienced surgeons, however, the need for multidisciplinary cooperation leads the radial group to a greater duration of surgery. It is of paramount importance that each bTME is discussed in the MDT and the surgical date and planning are organized together before the surgery to provide an efficient treatment.
Since the first report of robotic pelvic exenteration for LARC by Shin et al. [9], also other authors have published studies on multivisceral pelvic resections evaluating the role of robotics in this technically demanding surgery [10, 30, 31]. Hino et al. published a retrospective study on a large number of patients (n: 31) reporting optimal short-term outcomes of robotic-assisted multivisceral resection of rectal cancer invading or adhering to neighboring organs accessing the promising role of robotic surgery for such complex resections [10]. With low blood loss rate, no need for intraoperative transfusion, no unplanned conversion and low postoperative complication rate, they have reported the clinical benefits of robotic-assisted multivisceral resection10.
In the present study, blood loss was higher (Table 1) with seven patients (5.1%) requiring an intraoperative blood transfusion. However, this may be consequent to our different inclusion and exclusion criteria which is less strict than Hino et al. [10]. In their series, multivisceral resection with urinary diversion or reconstruction or total pelvic exenteration for complete tumor resection were not included in the study as were a candidate for open surgery due to no coverage for the robotic approach by the Japanese health insurance.
No unplanned conversion was reported in our series showing that the robotic approach is technically feasible in experienced hands and can overcome technical difficulties that may occur during a laparoscopic approach. This is important because in previous reports one of the main reasons for conversion (in laparoscopic series) was tumor fixation to adjacent pelvic organs or structures requiring extra-anatomic dissection with rates up to 21.2% [32,33,34,35]. Moreover, the 2013 Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines for laparoscopic resection of curable colon and rectum cancer recommended open surgery when laparoscopic en-bloc resection cannot be performed adequately for LARC with the suspected invasion of adjacent structures [36]. Our report, together with Hino et al. [10], strengthens the role of the robotic approach in the treatment of extended LARC and pushes forward a re-evaluation of surgical guidelines.
Postoperative complication rate is important to evaluate the safety of the surgical treatment. The radial group was the most affected by postoperative complications (n:13, 68%) compared to the lateral (n: 16, 53%) and the longitudinal (n: 37, 42%). Leakage and ileus were more frequent in the radial group (n:4, 21% and n: 6, 32%, respectively) compared to the lateral (n: 4, 13% and n: 6, 20%, respectively) and the longitudinal (n: 5, 6% and n: 19, 22%, respectively). The higher rate of complications of the radial group may be consequent to the involvement of complex anatomical districts (anterior genito-urinary and posterior sacral bone) and the cooperation of different multidisciplinary surgeons with possible divergent surgical skills on the robotic platform. Instead, both lateral and ISR dissections are single surgeon procedures.
Moreover, the relatively high rate of complication may be consequent to our colorectal database in which morbidity and mortality are prospectively maintained, on a weekly basis, since 2012 therefore, assuring high quality regarding this data.
The present study has a long term follow up with a median of 47 months (IQR, 31.5–66.5). This allows us to evaluate and discuss the oncological long-term outcomes of the three type of bTME.
Survival rates were good for the lateral and longitudinal group with 1- and 3-years OS of 96.6% and 84.8% (lateral) and 97.7% and 86.9% (longitudinal). OS was the worst for the radial group (1- and 3-years OS of 77.8% and 55.0%) reflecting the advanced biology of these cases compared to the other two types of bTME (p = 0.023).
Disease-free survival was not significantly different between the three types of bTME (p = 0.897).
Local recurrence occurred in 21 patients (15.3%) with a statistical difference between the three types of bTME (p = 0.041), with the lateral reporting up to 30% (seven lateral, one axial and one anterior).
In our series, the radial group reported the worst OS and the lateral group reported the worst LRFS.
The multivariate analysis showed that (y)pT, (y)pN and type of bTME were prognostic factors for OS, however, it was not possible to find significance for LRFS probably due to the relatively low number of cases. Interestingly there was no association between resection margin status and LR rate as reported by Peacock et al. [37].
The strength of this study is to report a wide experience on robotic-assisted bTME and to analyze three different types of bTME according to our “three degrees of dissection” definition of bTME rectal cancers. Clinical, pathological and oncological characteristics are reported together with long term oncological outcomes with a long-term follow-up.
However, this study has potential limitations. First, it is a retrospective analysis of a prospectively maintained database therefore, there could be a bias of selection associated with this type of analysis. Second, this study reports data only on an Asian population (South Korean), with a generally lower BMI, from a single institution. Third, a greater patient pool is needed to improve the statistical analysis for the identification of the prognostic factors for LRFS.
Each of the three groups of bTME may benefit from the robotic approach as for (1) allowing the surgeon a better dexterity for accessing the pelvic lateral node compartment to optimize the dissection; (2) enabling the surgeon to perform extensive operations, as for the radial group, with a minimally invasive approach with the possible better postoperative course; (3) providing a detailed anatomy of the deep pelvis, as for the longitudinal group, allowing a precise oncologically safe dissection with preservation of adjacent organs (e.g., nerves, urethra, vagina, and pelvic floor muscles) [9, 19]. However, further multicentric long term studies and eventually randomized controlled trials (comparing open, laparoscopic and robotic approach) are needed to confirm our results and to further highlight the benefits and limits of robotic surgery for LARC.
This study confirms the surgical and oncological safety of robotic-assisted bTME for LARC. To our knowledge, this is the first report evaluating bTME rectal cancer according to our definition of “three degrees of dissection”.
The results of the “Beyond TME Trial” (NCT02292641), completed in October 2019, are awaited by the colorectal surgical community to obtain a better detailed MRI imaging tumor assessment with a novel radiological staging classification for enhancing the selection of patients who may benefit an extended resection and improve the surgical planning.
Conclusions
Our “three degrees of dissection” (radial, lateral and longitudinal) is a novel classification of bTME rectal cancers. This study may justify the continuing application of the robotic approach to LARC with bTME dissection since short and long term outcomes are acceptable. Pathological T and N stage and type of bTME were prognostic factors for OS, however, no prognostic factors were identified for LRFS in this study. Further studies are required to confirm our results and strengthen the role of robotic-assisted surgery for extended LARC.
Code availability
Not applicable.
References
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery–the clue to pelvic recurrence? Br J Surg 69:613–616
Heald RJ, Moran BJ, Ryall RD et al (1998) Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 133:894–899
van Gijn W, Marijnen CA, Nagtegaal ID et al (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12:575–582
Beyond TMEC (2013) Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg 100:1009–1014
Lehnert T, Methner M, Pollok A et al (2002) Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg 235:217–225
Larsen SG, Wiig JN, Dueland S et al (2008) Prognostic factors after preoperative irradiation and surgery for locally advanced rectal cancer. Eur J Surg Oncol 34:410–417
Smith JD, Nash GM, Weiser MR et al (2012) Multivisceral resections for rectal cancer. Br J Surg 99:1137–1143
Kusters M, Austin KK, Solomon MJ et al (2015) Survival after pelvic exenteration for T4 rectal cancer. Br J Surg 102:125–131
Shin JW, Kim J, Kwak JM et al (2014) First report: Robotic pelvic exenteration for locally advanced rectal cancer. Colorectal Dis 16:O9-14
Hino H, Yamaguchi T, Kinugasa Y et al (2017) Robotic-assisted multivisceral resection for rectal cancer: short-term outcomes at a single center. Tech Coloproctol 21:879–886
Kumar NAN, Kammar P, Saklani A (2018) Minimal invasive approach for beyond total mesorectal excision/extended resections in rectal cancer. Mini-invasive Surg 2:19
Weiser MR (2018) AJCC 8th edition: colorectal cancer. Ann Surg Oncol 25:1454–1455
Kim SH, Park IJ, Joh YG et al (2008) Laparoscopic resection of rectal cancer: a comparison of surgical and oncologic outcomes between extraperitoneal and intraperitoneal disease locations. Dis Colon Rectum 51:844–851
Baek SJ, Kim SH, Kwak JM et al (2013) Selective use of preoperative chemoradiotherapy for T3 rectal cancer can be justified: analysis of local recurrence. World J Surg 37:220–226
Kusters M, Marijnen CA, van de Velde CJ et al (2010) Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol 36:470–476
von Elm E, Altman DG, Egger M et al (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349
Leong QM, Son DN, Cho JS et al (2011) Robot-assisted intersphincteric resection for low rectal cancer: technique and short-term outcome for 29 consecutive patients. Surg Endosc 25:2987–2992
Kim SH, Shin JW (2012) Robot-assisted intersphincteric resection. In: Schiessel R, Metzger P (eds) Intersphincteric Resection for Low Rectal Tumors. Springer, Vienna, pp 159–163
Piozzi GN, Park H, Choi TS et al (2020) Intersphincteric resection for low rectal cancer: a review on anatomy and surgical technique, oncologic and functional outcomes and the role of robotics. Turk J Colorectal Dis 30:76–85
Kim HJ, Choi GS, Park JS et al (2018) Selective lateral pelvic lymph node dissection: a comparative study of the robotic versus laparoscopic approach. Surg Endosc 32:2466–2473
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Siegel R, Burock S, Wernecke KD et al (2009) Preoperative short-course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: a multi-centre prospectively randomised study of the Berlin Cancer Society. BMC Cancer 9:50
Patel UB, Taylor F, Blomqvist L et al (2011) Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 29:3753–3760
Sauer R, Liersch T, Merkel S et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933
Glynne-Jones R, Wyrwicz L, Tiret E et al (2018) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:263
Mariathasan AB, Boye K, Giercksky KE et al (2018) Beyond total mesorectal excision in locally advanced rectal cancer with organ or pelvic side-wall involvement. Eur J Surg Oncol 44:1226–1232
Derici H, Unalp HR, Kamer E et al (2008) Multivisceral resections for locally advanced rectal cancer. Colorectal Dis 10:453–459
Harris DA, Davies M, Lucas MG et al (2011) Multivisceral resection for primary locally advanced rectal carcinoma. Br J Surg 98:582–588
Madoff RD (2006) Extended resections for advanced rectal cancer. Br J Surg 93:1311–1312
Malakorn S, Sammour T, Pisters LL et al (2017) Robotic Total Pelvic Exenteration. Dis Colon Rectum 60:555
Heah NH, Wong KY (2020) Feasibility of robotic assisted bladder sparing pelvic exenteration for locally advanced rectal cancer: a single institution case series. World J Gastrointest Surg 12:190–196
van der Pas MH, Haglind E, Cuesta MA et al (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Yamaguchi T, Kinugasa Y, Shiomi A et al (2016) Robotic-assisted vs conventional laparoscopic surgery for rectal cancer: short-term outcomes at a single center. Surg Today 46:957–962
Angelis N, Landi F, Vitali GC et al (2017) Multicentre propensity score-matched analysis of laparoscopic versus open surgery for T4 rectal cancer. Surg Endosc 31:3106–3121
Bretagnol F, Dedieu A, Zappa M et al (2011) T4 colorectal cancer: is laparoscopic resection contraindicated? Colorectal Dis 13:138–143
Zerey M, Hawver LM, Awad Z et al (2013) SAGES evidence-based guidelines for the laparoscopic resection of curable colon and rectal cancer. Surg Endosc 27:1–10
Peacock O, Waters PS, Bressel M et al (2019) Prognostic factors and patterns of failure after surgery for T4 rectal cancer in the beyond total mesorectal excision era. Br J Surg 106:1685–1696
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Conceptualization: GNP and SHK; Methodology: GNP and SHK; Formal analysis and investigation: GNP, T-HL; Writing—original draft preparation: GNP; Writing—review and editing: J-MK, JK, SHK; Supervision: SHK.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was formally approved by the Ethics Committee of the institution (Institutional Review Board) where it was developed. Animals were not used.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was formally approved by the Ethics Committee of the institution (Institutional Review Board) where it was developed. Animals were not used.
Consent to participate
Informed consent is not required for this type of study.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Informed consent
Informed consent is not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piozzi, G.N., Lee, TH., Kwak, JM. et al. Robotic-assisted resection for beyond TME rectal cancer: a novel classification and analysis from a specialized center. Updates Surg 73, 1103–1114 (2021). https://doi.org/10.1007/s13304-020-00898-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-020-00898-0