Abstract
Background
The Enhanced Recovery After Surgery (ERAS) program has been shown to reduce length of stay (LOS) in colorectal surgical patients in randomized trials. The impact outside of trial settings, or in subgroups of patients excluded from trials such as individuals with diabetes, is uncertain. We conducted this study to evaluate the impact of ERAS implementation in Alberta, Canada.
Methods
This is a retrospective cohort study and interrupted time series analysis using linked administrative data to examine LOS and postoperative outcomes in the 12 months pre- and post-implementation of ERAS in 2013 for all adults undergoing elective colorectal surgery.
Results
Of 2714 patients (mean age 60.4 years, 55% men) with similar demographics and comorbidity profiles in the pre/post-ERAS time periods, LOS was significantly shorter post-ERAS (8.5 vs. 9.5 days, p = 0.01; − 0.84 days [95% CI − 0.04 to − 1.64 days] after adjustment for age, sex, Charlson comorbidity score, procedure type, surgical approach, and hospital). However, interrupted time series demonstrated no significant level change (p = 0.30) or change in slope (p = 0.63) with ERAS implementation, consistent with continuation of an underlying secular trend of reductions in LOS over time. There were no significant differences (in multivariate analysis or ITS) in risk of 30-day death/readmission (14.3% post vs. 13.5% pre-ERAS, aOR 1.12, 95% CI 0.89–1.40), 30-day death/ED visit (27.2% post vs. 30.0% pre, aOR 0.93, 95% CI 0.78–1.10), or 30-day death/readmission/ED visit (27.8% post vs. 30.6% pre, aOR 0.93, 95% CI 0.78–1.10). The 428 patients with diabetes had longer LOS but exhibited no significant difference post- versus pre-ERAS (10.7 vs. 11.6 days, p = 0.53; p = 0.56 for level change and p = 0.66 for slope change on ITS).
Conclusion
Although there was a secular trend toward decreasing LOS over time in Alberta, ERAS implementation was not associated with statistically significant changes in LOS or postoperative outcomes for all colorectal surgery patients or for those with diabetes. Our study highlights the importance of evaluating system changes (for both uptake and outcomes) rather than assuming trial benefits will translate directly into practice. Interventions to improve LOS and postoperative outcomes for patients with diabetes undergoing colorectal surgery are still needed even in the ERAS era.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
A multimodal care approach to improve surgical outcomes (the Enhanced Recovery After Surgery [ERAS®] program) has demonstrated promising results and is being implemented across many centers worldwide [1,2,3]. The ERAS® program includes 22 components of pre-, intra-, and postoperative care [1, 4]. A meta-analysis of 16 randomized controlled trials (RCTs) of enhanced recovery programs (including, but not limited to ERAS®) with 2376 patients reported an approximately 40% reduction in postoperative complications and a 2.3-day reduction in length of stay (LOS) without any increase in readmission rates [5]. However, as adherence to the ERAS program elements is a key driver of outcomes [6] and is likely to vary outside of the controlled setting of an RCT, it is important to confirm the benefits seen in RCTs do translate into clinical practice when ERAS® is implemented in real-world hospitals. Moreover, although they comprise a substantial proportion of the surgical population, patients with diabetes have not been included in previous trials of enhanced recovery programs and it is unclear whether they would obtain similar benefits compared to patients without diabetes [7].
In the Canadian province of Alberta, ERAS® was implemented at the main colorectal surgical sites starting in 2013 for all patients, including those with diabetes, thereby precluding conducting an RCT. However, this health policy natural experiment allows us to evaluate the impact of ERAS® on postoperative LOS and outcomes (postoperative complications and 30-day death, readmission, or ED visits after discharge) in an entire healthcare system and for those individuals with diabetes using an interrupted time series (ITS) analysis: a quasi-experimental design that allows the evaluation of system-wide effectiveness while accounting for any underlying secular trends.
Methods
Setting
The province of Alberta has a single integrated healthcare system, providing universal coverage for 4.2 million people. The six hospitals where ERAS was implemented are located in Calgary (2) and Edmonton (4). This study was approved by the Health Research Ethics Board at the University of Alberta, and de-identified linked data from the Discharge Abstract Database (DAD), the National Ambulatory Care Reporting System (NACRS), and the provincial registry for vital status of Albertans were used.
Cohort
All elective hospitalizations of adults for colorectal surgery at the six hospitals were identified in the 12 months pre- and post-ERAS implementation at each site. We excluded patients already in-hospital for other medical conditions, or those who did not have their surgery on the day of admission. All patients undergoing elective colorectal surgery were approached and actively followed by ERAS® case managers who monitored for complications via chart review and telephone follow-up (complications captured in the DAD were also collected via chart review by trained nosologists using the same procedures pre/post-ERAS®).
Covariates
Comorbidities and the Charlson comorbidity score were defined using International Classification of Disease ICD-10 codes from the index hospitalization and all hospitalizations, ED visits, or ambulatory care visits in the 2 years prior to their index admission, using definitions previously validated in Alberta databases [8,9,10]. Rural residence was also captured given the well-recognized association between location, socioeconomic status, and colorectal cancer outcomes [11].
Outcomes
Primary outcome was acute LOS (number of days in acute care during the index hospitalization, but not counting days after transfer to other hospitals or rehabilitation facilities). Secondary outcomes included total LOS (all days during index hospitalization plus all days in transfer hospitals); postoperative complications (ICD-10 and Canadian Classification of Health Intervention (CCI) codes listed in “Appendix”; these have been shown to be sensitive for detecting Clavien class III or greater postoperative complications [12,13,14], i.e., those that are most clinically relevant); 30-day death/readmission; 30-day death/ED visits; and 30-day death/readmission/ED visits after discharge from the index hospitalization [12].
Analysis
Patient characteristics were summarized using proportions and means and compared between the pre- and post-ERAS periods (for all six sites combined) using Chi-square tests and t test, respectively. First, a univariate analysis was conducted comparing outcomes in the pre- and post-ERAS periods, as well as the change adjusted for age, sex, Charlson score, procedure type, surgical approach (laparoscopic or open), and hospital site (odds ratios for binary outcomes and difference in means for continuous outcomes).
Finally, because pre/post-analyses do not account for secular trends or serial dependencies, we conducted an interrupted time series (ITS) analysis to further evaluate the impact of ERAS implementation. A time series was created using the 12 months prior and the 12 months after the month of implementation at each hospital. Outcomes were summarized using bimonthly periods to reduce variability due to small sample size within each month, resulting in six time points both pre- and post-ERAS implementation. Autoregressive integrated moving average (ARIMA) models were initially considered, but after confirming stationarity and lack of autocorrelation using appropriate methods, our final ITS analysis was modeled using linear regression. All analyses were repeated in the subset of patients with diabetes.
Two sensitivity analyses were undertaken. In the first, we only examined data for patients in the post-ERAS phase clearly identified as having consented to and received the ERAS program (a form of “on-treatment analysis” which should maximize any benefit signal for ERAS as opposed to our primary “intention-to-treat analysis” including all patients). In the second, we excluded patients undergoing revision surgery in either timeframe (to focus on patients felt to be undergoing more complicated surgeries—a “higher-risk cohort analysis”).
All statistical analyses were performed using SAS version 9.4 (Cary, NC) and R version 3.3.3 (Vienna, Austria).
Results
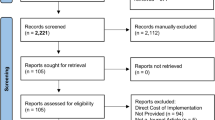
Our cohort consisted of 2714 patients (mean age 60.4 years, 55% men) who underwent elective colorectal surgery in the 12 months before and after implementation of ERAS at the six participating hospitals (Fig. 1). Patient demographics and comorbidity profiles were very similar in the pre/post-ERAS time periods (Table 1), although there were more colon and laparoscopic surgeries done post-ERAS and more revision surgeries pre-ERAS. The 428 patients with diabetes in our cohort were older and had higher comorbidity profiles than those without diabetes, but there were no appreciable differences between those in the pre- or post-ERAS periods (data available from first author on request). Baseline characteristics within each hospital were similar pre- and post-ERAS, overall and for patients with diabetes (data available from first author on request).
For all elective colorectal surgery patients, LOS was significantly shorter post-ERAS (acute LOS 8.5 days vs. 9.5 days, p = 0.01; total LOS 9.4 days vs. 10.8 days, p = 0.03), and these differences persisted after adjustment for age, sex, Charlson score, procedure type, surgical approach, and hospital (− 0.84 days [95% CI − 0.04 to − 1.64 days] for acute LOS and − 1.15 days [95% CI − 0.13 days to − 2.44 days] for total LOS)—Table 2. However, this appeared to represent continuation of a secular trend since the ITS demonstrated no significant level change (p = 0.30 for acute LOS and p = 0.42 for total LOS) or change in slope (p = 0.63 and p = 0.91, respectively) with ERAS implementation (Table 3, Fig. 2).
There was no significant difference in 30-day death/readmission/ED visit (27.8% post vs. 30.6% pre, aOR 0.93, 95% CI 0.78 to 1.10), or any of the other composite outcomes—Table 3. The ITS confirmed that ERAS implementation was not associated with any significant level changes in any of the 30-day outcomes—Table 3.
Complications during hospitalization were slightly higher post-ERAS (38.8 vs. 36.1%, aOR 1.22, 95% CI 1.03 to 1.43), with the most common being postoperative intestinal obstruction or peritoneal adhesions. However, this was not significantly associated with ERAS implementation (p = 0.60 for a level change and p = 0.18 for a slope change) and appeared to be due to changes in types of surgery over time as excluding revision surgeries in both time frames ameliorated the apparent hazard (38.4% post vs. 38.3% pre, aOR 1.12, 95% CI 0.94 to 1.34).
Mortality within 30 days of admission was not significantly different between the pre- and post-ERAS phases: 14 (1%) versus 20 (1.5%) in all patients (p = 0.27). In-hospital mortality also did not differ post versus pre (1.0 vs. 0.6% overall).
Our first sensitivity analysis (the “on-treatment analysis”) was restricted to the 83% of patients confirmed as having received ERAS, who were identified using the ERAS Interactive Audit System (EIAS) which captures all ERAS patients. There appeared to be a selection bias in that those patients explicitly enrolled in ERAS were less sick than patients not enrolled in ERAS (mean Charlson 2.7 vs. 3.8, 56.2% open surgical procedure vs. 73.8%, 0 in-hospital deaths vs. 5.8% in-hospital deaths, and 35.5% perioperative complication rate vs. 55.1%). However, even those patients confirmed to have received ERAS did not demonstrate a statistically significant improvement in outcomes compared to the pre-ERAS phase in multivariate analyses or ITS: 30-day death/readmission (12.5% post vs. 13.5% pre-ERAS, aOR 0.97, 95% CI 0.76–1.24, ITS p = 0.90), 30-day death/ED visit (25.7% post vs. 30.0% pre, aOR 0.86, 95% CI 0.72–1.04, ITS p = 0.21), or 30-day death/readmission/ED visit (26.2% post vs. 30.6% pre, aOR 0.87, 95% CI 0.72–1.04, ITS p = 0.18). Although LOS was 1.2 (95% CI 0.4 to 2.1) days shorter for those patients confirmed as being exposed to ERAS, the ITS demonstrated no statistically significant level change after accounting for underlying temporal trends (ITS p = 0.37).
Our second sensitivity analysis excluding patients undergoing revision (the “high-risk cohort analysis”) also did not identify any significant association between ERAS implementation and changes in outcomes or LOS (results available from first author on request).
Diabetes subgroup
Diabetic patients had a longer LOS but no significant difference between post- and pre-ERAS (acute LOS 10.7 vs. 11.6 days, p = 0.53; total LOS 12.0 vs. 13.4 days, p = 0.42; adjusted differences − 0.63 and − 0.89 days, respectively, with nonsignificant confidence intervals, Table 3) and no significant ITS level changes (p = 0.56 and p = 0.28) or slope changes (p = 0.66 and p = 0.25) (data available from first author on request). While the diabetes patients exhibited higher event rates for all four outcomes (Table 3), there were no statistically significant adjusted differences post- and pre-ERAS and ITS confirmed no level changes—data available from first author on request.
Mortality within 30 days of admission was not significantly different between the pre- and post-ERAS phases: 3 (1.4%) versus 4 (1.9%) (p = 0.71). In-hospital mortality also did not differ post versus pre (1.4 vs. 0.5%).
Discussion
Although we found a significant decrease in LOS over time in adults undergoing elective colorectal surgery in Alberta, this appeared to be a temporal trend and was not statistically significantly associated with ERAS implementation. We have seen similar declines in LOS for hospitalizations on medicine wards in Alberta in recent years too (likely attributable to more ready access to diagnostic imaging resources and better outpatient resources to facilitate earlier discharge including home intravenous therapy teams, wound care teams, and extra homecare nursing capacity) [15]. On the other hand, we did not find any evidence of increased risk associated with ERAS implementation and instead observed nonsignificant decreases in post-discharge deaths/readmissions/ED visits post versus pre-ERAS. We found that patients with diabetes were a higher-risk group, with longer LOS and higher event rates for all outcomes, but there were no significant differences in any outcomes after ERAS.
How do our findings compare to the existing literature?
There have been no RCTs evaluating ERAS® per se, but RCTs evaluating other enhanced recovery and fast-track programs with elements that overlap with ERAS® have reported reductions in LOS and postoperative complications without an increase in 30-day readmissions [5, 16]. The majority of RCTs included in the previously published meta-analyses were small, and more than half of the trials were at moderate to high risk of bias [5]. Since the publication of the ERAS® colorectal protocol in 2005, several observational studies have reported reductions in LOS and postoperative complications [2, 3, 17,18,19]. However, before–after studies and cohorts without control groups carry substantial risk of bias and cannot be used to infer causality. Although a recent publication from 20 hospitals in Kaiser Permanente Northern California reported greater reductions in LOS and postoperative complications in the year after implementation of ERAS for colorectal resection patients, their adjustment for temporal trends involved comparing outcome trends in ERAS patients to those in contemporaneous surgical comparators [2]. However, perusal of their supplementary tables raises questions about whether the comparators really were an appropriate choice: They were almost a decade younger than the ERAS patients, less than 10% had lower GI surgery (three-fourths had gastrectomy, nephrectomy, or hernia repairs), and their LOS (2.2 days vs. 5.1 days) and complication rates (6.9 vs. 18.1%) were already substantially lower than LOS for colorectal surgery patients–meaning that the size of any potential outcome improvements for the comparator patients was markedly restricted.
A recent uncontrolled before–after study of the first 15-month experience with ERAS in two Alberta hospitals reported a mean LOS of 9.8 days (n = 130) pre-ERAS, compared to 7.5 days (n = 697) post-ERAS (p < 0.0001) [18]. This is comparable to the acute LOS observed in our analysis of all six Alberta sites. The two-site audit reported that compliance with ERAS elements increased during the first 3 months of implementation and then stayed constant thereafter with median compliance of 60% up to 15 months out at those two early adopter sites. On the other hand, median LOS in multicenter international ERAS® registries was 4–6 days—much shorter than that observed in our cohort—but compliance with ERAS elements was higher [2, 3, 17]. Lower compliance with components of the ERAS program could explain the apparent lack of effect of ERAS implementation in Alberta (either overall or even in the “on-treatment” analysis) given that a dose–response relationship between compliance with ERAS elements and reduction in postoperative outcomes has been suggested in several studies and optimal outcomes were observed when compliance was > 70% [3, 6, 19].
In the large multicenter international ERAS registry [3], postoperative complications were reported in 40.3% of patients undergoing elective colorectal cancer surgery, comparable to the risk observed in our cohort (38.8% in all patients and 41.9% in the diabetes subgroup). Enhanced recovery processes in general have been reported to reduce complications [1, 2, 5]. Although no reduction was observed in our study, this might again be related to lower compliance to the ERAS program (only 60% in the analysis by Nelson et al. of the two hospitals who were early adopters of ERAS in Alberta and very likely lower in the other four later adopting hospitals since early adopters tend to exhibit highest adherence rates) [18], or the predominance of open surgical approaches in Alberta—a well-established risk factor for ileus and small bowel obstruction [20, 21] (about two-thirds of colorectal surgeries compared to 53% in the largest case series completed to date) [3].
What about the diabetes subgroup?
Evidence is scarce examining the impact of ERAS in individuals with diabetes, and small observational studies have demonstrated inconsistent results [7]. We found an unexpected strong trend toward an excess in postoperative complications was observed in the diabetes group post-ERAS, although not statistically significant (aOR 1.49, 95% CI 0.98 to 2.27). This was related to higher gastrointestinal complications: peritoneal adhesions (12 [5.6%] pre-ERAS vs. 22 [10.2%] post-ERAS) and small bowel obstructions (17 [8%] vs. 24 [11.2%]), with the latter associated with ileus in 96% of cases. This was unexpected given that these particular complications have been shown to occur less frequently with early nutrition postoperatively, removal of gastric tubes, less use of opioids, and early mobility—all key components of the ERAS program. Whether carbohydrate loading preoperatively—part of the ERAS—has an impact is unknown, and whether hyperglycemia perioperatively had an influence needs to be investigated further in an RCT (which we have initiated in Alberta). Another potential explanation may be a progressive increase in reporting of complications over time (i.e., “up-coding” where there is not a change in actual complication rates, just an increase in recognition and/or mention in discharge summaries).
Strengths and limitations
Strengths of our study include that the intervention of interest, ERAS, occurred independent of other changes over time, and by using routinely collected administrative health data, there was no influence of ERAS or our study on data collection for the outcomes of interest and no possibility of a Hawthorne effect. The primary outcomes examined were objective and derived from routinely collected health data using validated ICD-10 codes in both the pre- and post-ERAS periods. Given that this study was undertaken in a real-world setting, it carries stronger external validity compared to an efficacy RCT and allows assessment of the longitudinal impact of ERAS. Our analysis also meets criteria for a high-quality design for evaluation of health system changes: interrupted time series has been recognized by the Cochrane Effective Practice and Organization of Care methods group (http://epoc.cochrane.org/) as the strongest study design after RCTs for evaluation of organizational interventions in healthcare services [22].
A number of limitations, however, need to be considered. First and foremost, individual patient data on adherence to the ERAS program were not available precluding an examination of whether the lack of apparent effect was due to non-adherence or true negative effects. However, no signal was detected in either the primary “intention-to-treat” or the sensitivity “on-treatment” analyses. Second, our ITS is not as robust as an RCT design, and residual confounding cannot be excluded. However, we also conducted multivariate analyses adjusting for baseline characteristics and type of surgery to compare post versus pre-outcomes, and the fact that there were no significant changes in baseline characteristics between the pre- and post-ERAS periods is reassuring for our ITS, as is the fact that our results were similar in the “high-risk cohort” analysis where differences would be most likely to be seen. Identification of covariates was based on ICD codes which, although validated in Alberta [8, 9], are subject to potential misclassification bias. Our ITS had 12 data points in total, and this may result in less power to detect an effect; however, these were equally distributed pre- and post-ERAS, there was no autocorrelation, and the reported effect in the published RCTs was large, suggesting that our study was adequately powered [23, 24]. Regardless, repeating this analysis in a couple of years with additional patients and as the hospitals optimize adherence to ERAS might detect a treatment effect that was too small to find in this study.
Conclusion
Our large cohort study demonstrated a consistent downward trend in LOS over time in Alberta but little change in LOS or perioperative outcomes statistically significantly associated with ERAS implementation. This may well be due to lower adherence with ERAS elements in the studied hospitals, although we do not have the necessary information to answer that question. Moreover, our data illustrate that individuals with diabetes remain at high risk, even after implementation of an ERAS program, suggesting the need for further interventions to improve perioperative outcomes for patients with diabetes undergoing colorectal surgery. Our study highlights that we cannot assume that the benefits suggested in RCTs will always be accrued in routine clinical practice and the importance of prospectively evaluating any changes in care delivery models at a system level.
References
Ljungqvist O, Scott M, Fearon KC (2017) Enhanced recovery after surgery. A review. JAMA Surg 152:292–298
Liu VX, Rosas E, Hwang J, Cain E, Foss-Durant A, Clopp M et al (2017) Enhanced recovery after surgery program implementation in 2 surgical populations in an integrated health care delivery system. JAMA Surg 152:e171032
ERAS Compliance Group (2015) The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 261(6):1153–1159
Gustafsson UO, Scott MJ, Schwenk W et al (2012) Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS®) society recommendations. Clin Nutr 31(6):783–800
Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M (2014) Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 38(6):1531–1541. https://doi.org/10.1007/s00268-013-2416-8
Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygen J (2011) Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 146(5):571–577
Albalawi Z, Laffin M, Gramlich L, Senior P, McAlister FA (2017) Enhanced recovery after surgery (ERAS(®)) in individuals with diabetes: a systematic review. World J Surg 41:1927–1934. https://doi.org/10.1007/s00268-017-3982-y
Quan H, Sundararajan V, Halfon P et al (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–1139
Quan H, Li B, Saunders LD et al (2008) Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 43(4):1424–1441
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Aarts MJ, Lemmens VEPP, Louwman MWJ, Kunst AE, Coebergh JWW (2010) Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer Oxf Engl 1990 46(15):2681–2695
Ma C, Crespin M, Proulx M-C et al (2012) Postoperative complications following colectomy for ulcerative colitis: a validation study. BMC Gastroenterol 12:39
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Rosenthal R, Hoffmann H, Clavien P-A, Bucher HC, Dell-Kuster S (2015) Definition and classification of intraoperative complications (CLASSIC): Delphi study and pilot evaluation. World J Surg 39(7):1663–1671. https://doi.org/10.1007/s00268-015-3003-y
McAlister FA, Bakal JA, Majumdar SR et al (2014) Safely and effectively reducing inpatient length of stay: a controlled study of the General Internal Medicine Care Transformation Initiative. BMJ Qual Saf 23(6):446–456
Lau CSM, Chamberlain RS (2017) Enhanced recovery after surgery programs improve patient outcomes and recovery: a meta-analysis. World J Surg 41:899–913. https://doi.org/10.1007/s00268-016-3807-4
Gillissen F, Hoff C, Maessen JMC et al (2013) Structured synchronous implementation of an enhanced recovery program in elective colonic surgery in 33 hospitals in The Netherlands. World J Surg 37(5):1082–1093. https://doi.org/10.1007/s00268-013-1938-4
Nelson G, Kiyang LN, Crumley ET et al (2016) Implementation of enhanced recovery after surgery (ERAS) across a provincial healthcare system: the ERAS Alberta Colorectal Surgery Experience. World J Surg 40(5):1092–1103. https://doi.org/10.1007/s00268-016-3472-7
Thiele RH, Rea KM, Turrentine FE, Friel CM, Hassinger TE, Goudreau BJ et al (2015) Standardization of care: IMpact of an Enhanced Recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg 220:430–443
Schnüriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D (2011) Prevention of postoperative peritoneal adhesions: a review of the literature. Am J Surg 201(1):111–121
Okabayashi K, Ashrafian H, Zacharakis E et al (2014) Adhesions after abdominal surgery: a systematic review of the incidence, distribution and severity. Surg Today 44(3):405–420
Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE (2003) Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care 19(4):613–623
Zhang F, Wagner AK, Ross-Degnan D (2011) Simulation-based power calculation for designing interrupted time series analyses of health policy interventions. J Clin Epidemiol 64(11):1252–1261
Bernal JL, Cummins S, Gasparrini A (2017) Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 46(1):348–355
Acknowledgements
This work was supported by the Alberta Strategy for Patient Oriented Research Support Unit Data Platform. The authors thank Mr. Edwin Rogers, M.A. (Senior Analyst, Clinical Analytics, Alberta Health Services) for assistance with colorectal surgery case definitions and logic, as well as access to the patient lists maintained by the ERAS team for cross-validation with our administrative data. They also extend their thanks to Jeff Bakal, Ph.D., P.Stat (Alberta SPOR Support Unit, University of Alberta) for assistance with study design and statistical methods and Peter Faris, Ph.D. (Director, Research Facilitation, Analytics, DIMR, Alberta Health Services) for statistical review. Zaina Albalawi and Erik Youngson had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest relating to this study.
Appendix
Appendix
ICD-10 codes used to define index hospitalization complications [13]
Category | Complications | ICD-10 |
|---|---|---|
Gastrointestinal | Small bowel obstruction, anastomotic stricture (include peritoneal adhesions), pouch leak pouch failure, bowel perforation, ileus, ischemic bowel, GI bleeding (also include other hemorrhage and hemorrhagic conditions), ileostomy/colostomy complication or malfunction, digestive organ disorders (include acute hepatic failure and acute pancreatitis), other GI complications (include pneumatosis) | K22.8, K25.0, K25.2, K25.4, K25.6, K26.0, K26.1, K26.2, K26.4, K26.5, K26.6, K27.0, K27.2, K27.4, K27.6, K28.0, K28.2, K28.4, K28.6, K29.0, K55.0, K55.9, K56.0, K56.5, K56.6, K56.7, K62.5, K63.1, K63.8, K66.0, K65.5, K65.6, K72.0, K72.9, K85, K91.3, K91.4, K91.8, K91.9, K92, T79.2, T81.0, T88.8 |
Wounds | Fistula, hematoma/seroma, wound dehiscence and delayed wound healing, Iatrogenic injuries (include foreign body accidentally left during procedure) and pressure ulcer. | K60.3, K60.4, K60.5, K63.2, K82.9, K83.2, L89, N36.0, N82.4, T81.2, T81.3, T81.5, T81.8, 1.OT.52.DA, 1.OT.56.DA, 1.OT.70.LA,1.OW.80, 2.OT.70.LA |
Infections | Sepsis and bacteremia, abscess, wound infection, urinary tract infection, pneumonia and empyema, other infections (include peritonitis, and bacterial skin and subcutaneous tissue infection). | A40, A41, A49, B95, B96, J10.0, J11.0, J12, J13, J14, J15, J16, J17, J18, J69.0, J85, J86, K61, K63.0, K65, L03, L04, N10, N12, N15.1, N15.9, N30.0, N30.9, N39.0, R78.8, T79.3, T80.2, T81.4, T81.6, T82.7, T83.6, T85.7 |
Renal and endocrine | Acute renal failure, fluid and electrolyte disorders (include hypokalemia), severe endocrine disorders (include adrenal disorders and hypoglycemic coma), retention of urine (include atony of bladder), other urinary complication (include urinary obstruction) | E15, E272, E86, E87, N13.9, N17, N19, N31.2, N99.0, N99.9, R33 |
Cardiovascular disorders | Thrombosis/embolism, myocardial infarction, cardiac arrest, hypotension or shock, cardiac arrhythmias (exclude tachycardia), heart failure, other cardiovascular complication (include atherosclerotic heart disease, and angina) | I21, I26, I46, I48, I49, I50, I74, I80, I81, I82, I95.0, I95.2, I95.9, I97.8, I97.9, R57, T79.0, T80.0, T80.1, T81.1, T81.7, T88.2 |
Pulmonary | Acute respiratory failure, hypoxia, pleural effusion and pulmonary edema, pneumothorax and atelectasis, other pulmonary complications (include asthma, extubation failure, and difficulty breathing) | J80, J81, J90, J91, J93, J95.5, J95.8, J95.9, J96.0, J96.9, J98.1, R09 |
Neurological disorders | Cerebrovascular disease, neurological disorders (psychoses/delirium/seizure), disorders/complications of nervous system (include neuropathies) | F05, F13, F15, F19, G45, G46, G81, G82, G83, G93.1, G93.6, G97.0, G97.1, G97.8, G97.9, I63, I65 |
Rights and permissions
About this article
Cite this article
AlBalawi, Z., Gramlich, L., Nelson, G. et al. The Impact of the Implementation of the Enhanced Recovery After Surgery (ERAS®) Program in an Entire Health System: A Natural Experiment in Alberta, Canada. World J Surg 42, 2691–2700 (2018). https://doi.org/10.1007/s00268-018-4559-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4559-0