Abstract
Background
Complete mesocolic excision with central vascular ligation is a standard advanced technique for achieving favorable long-term oncological outcomes in colon cancer surgery. Clinical evidence abounds demonstrating the safety of high ligation of the inferior mesenteric artery (IMA) for sigmoid colon cancer but is scarce for descending colon cancer. A major concern is the blood supply to the remnant distal sigmoid colon, especially for cases with a long sigmoid colon. We sought to clarify the safety and feasibility of high ligation of the IMA in surgery for descending colon cancer using indocyanine green (ICG) fluorescence imaging.
Methods
In this prospective single-center pilot study, we examined 20 patients with descending colon cancer who underwent laparoscopic colectomy between April 2018 and September 2019. Following full mobilization and division of the proximal colonic mesentery, we temporarily clamped the root of the IMA and performed ICG fluorescence imaging of the blood flow to the sigmoid colon. The postoperative anastomosis-related complications (primary endpoint) and length of viable remnant colon, and the number of lymph nodes retrieved (secondary endpoints) were evaluated and compared with historical controls who underwent conventional IMA-preserving surgery (n = 20).
Results
Blood flow reached 40 (17–66) cm retrograde from the peritoneal reflection, even after IMA clamping. Accordingly, IMA high ligation was performed in all cases. No anastomotic anastomosis-related complications occurred in each group. Retrieved total lymph nodes were higher in number in the ICG-guided group than in the conventional group (p = 0.035). Specifically, more principal nodes were retrieved in the ICG-guided group, compared with the conventional group (p = 0.023). However, the distal margin was not as long compared with the conventional group.
Conclusion
We demonstrated the safety and feasibility of high ligation of the IMA for descending colon cancer without sacrificing additional distal colon using fluorescence evaluation of blood flow in the remnant colon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lymph node dissection plays an important role in surgical resection of colorectal cancer for achieving long-term survival. Regional lymph nodes in colorectal cancer have been classified into epiploic, intermediate mesocolonic, and principal lymph node groups. Total mesorectal excision for rectal cancer is well known and widely implemented as a feasible and important procedure in extending overall survival [1]. Similarly, complete mesocolic excision (CME) with central vascular ligation (CVL) has been considered important for achieving favorable long-term oncological outcomes in colon cancer [2]. Although the effects of CME with CVL have been reported for right hemicolectomy and sigmoidectomy, there is a paucity of evidence for descending colectomy, possibly due to the low incidence of cancer of the descending colon (5% of all colorectal cancers) [2, 3]. In complete CME/CVL for advanced left-sided colonic cancer, high ligation of the IMA is needed. However, concerns have been raised about the blood supply to the remnant sigmoid colon. This is especially true for patients with a long sigmoid colon, because blood flow from the internal iliac artery system has conventionally been considered not to extend beyond the level of the sacral promontory [4, 5].
Therefore, preservation of the inferior mesenteric artery/superior rectal artery (IMA/SRA) is preferred in surgery for cancer of the descending colon. However, preservation of the IMA/SRA is at odds with the concept of CVL and concerns have been raised about reduced lymph node yield compared with high ligation of the IMA. However, even when the IMA is ligated, resection of the sigmoid colon up to the sacral promontory, where blood flow is typically sufficient, is a somewhat overly excessive surgery, especially for patients with a long sigmoid colon.
In other words, in performing substantial lymphadenectomy, including blood supply preservation and removal of an appropriate length of colon, it is essential to determine the blood perfusion to the descending and sigmoid colon. Here, we used indocyanine green (ICG) fluorescence as a form of live imaging to visualize blood perfusion, which is currently a topic of great interest in the field of surgical navigation, especially in laparoscopic surgery. Following intravenous injection, ICG binds to plasma proteins and emits fluorescence at a peak wavelength of 845 nm. It has been reported that ICG fluorescence imaging is useful for determining the transection line in laparoscopic colorectal surgery, and that intraoperative fluorescence imaging reduced anastomotic leakage, compared with a control group [6]. In the present study, we sought to demonstrate the safety and effectiveness of high ligation of the IMA for descending colectomy using ICG fluorescence imaging.
Patients and methods
Study population
We enrolled 20 patients who were diagnosed as having colon cancer located in the descending colon (including the sigmoid-descending colon junction) and underwent elective laparoscopic surgery between April 2018 and September 2019 at Fukuoka University Hospital. The exclusion criteria were a history of previous colorectal surgery and a history of hypersensitivity reaction to iodinated contrast media. The study protocol was approved by the institutional review board of Fukuoka University, Fukuoka, Japan, and was registered in the UMIN Clinical Trials Registry (UMIN000035450; https://www.umin.ac.jp/ctr/index.htm). Written informed consent was obtained from all patients for the use of their clinical data.
Preoperative evaluation
Patients underwent total colonoscopy and contrast-enhanced computed tomography for a definitive diagnosis of colon cancer and to determine the clinical stage and extent of the tumor, nodal status of metastasis, and spread to distant sites. For all patients, we diagnosed adenocarcinoma by histological examination of a biopsy specimen. The entire tumor was located in the descending colon or at the SDJ, and so endoscopic tattooing of the tumor borders was performed to localize the tumor for easy identification during surgery. In all cases, we confirmed the main feeding vessel to be the IMA using computed tomography angiography.
Surgical procedure
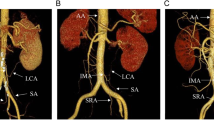
A schematic of the surgical procedure is shown in Figs. 1 and 2. Laparoscopic surgery was performed under general anesthesia with or without epidural anesthesia in all cases. First, we divided the greater omentum from the transverse colon and the patient was repositioned in a slight Trendelenburg position. Next, we dissected the left mesocolon via the medial approach from the right side of the inferior mesenteric vein, keeping the mesocolon wrapped in fascia and then the mesentery was divided toward the oral side of the colon to block blood flow from the oral side of the mesentery (Figs. 1A and 2A,B). The rectosigmoid mesentery was then mobilized via the medial approach up to the root of the IMA, which was skeletonized (Fig. 1B) and then clamped with a temporary clip to block blood flow (Fig. 2C). Under these conditions, where blood flow in the descending colon was maintained only via the internal iliac artery, we intravenously injected 5 mg of ICG to evaluate the blood flow. Blood flow was observed using an endoscopic ICG near-infrared fluorescence imaging system (1588 AIM camera system; Stryker Corporation, Kalamazoo, MI). We measured the distance from the peritoneal reflection (PR) to the tumor (Tu), from the PR to the promontorium (P), from the PR to the planned distal margin (DM) (usually defined as 10 cm from the tumor on the anal side), and from the PR to the upper margin of the area of contrast limit (CL), which is the oral point marking the extent of the dye. We also measured the duration from the time the common iliac artery bifurcation was imaged to the time the sigmoid colon at the level of the sacral promontory was imaged (Figs. 1C and 2D).
Study protocol 1 A The splenic flexure is fully mobilized, and the mesentery is divided to the oral side of the colon to block the blood flow from the oral side of the mesentery. B The rectosigmoid colon is mobilized and the root of the inferior mesenteric artery (IMA) is skeletonized. C The root of the IMA is clamped and indocyanine green is injected intravenously. Measurements are obtained for the distance between the peritoneal reflection (PR) and the promontorium (P), the planned distal margin (DM), contrast limit (CL), and the tumor (Tu)
Study protocol 2 A The proximal division line (PM) is determined at an appropriate length from the tumor. B Following mobilization of the splenic flexure, the colonic mesentery is divided to block the blood flow from the oral side. C The root of the inferior mesenteric artery (IMA) is clamped after the rectosigmoid mesentery is mobilized. LCA, left colic artery; SRA, superior rectal artery D Indocyanine green is injected intravenously and blood flow to the sigmoid colon is evaluated using fluorescence imaging
If the blood flow of the planned distal margin was good, we performed high ligation of the IMA and anastomosis of the proximal and distal colon; if not, the IMA/SRA was preserved. The anastomosis method used (functional end-to-end anastomosis [FEEA] or double stapling technique [DST]) was at the discretion of the surgeon. We again confirmed blood supply to the oral and anal sides of the colon just before the anastomotic procedure using ICG fluorescence as well as a conventional method (e.g., color of the colon).
Statistical analysis
The primary endpoint of this study was the rate of anastomosis-related complications, specifically leakage and stenosis. Secondary endpoints included the length of viable remnant colon, the number of lymph nodes retrieved, the length of resected intestine, and the rate of high ligation of the IMA. To evaluate the feasibility of this ICG-guided strategy with conventional IMA-preserving surgery, we compared our data with those of 20 consecutive patients who underwent left colectomy with IMA/SRA preservation in our institute between 2014 and 2017.
We performed statistical analyses using JMP 15.1.0 statistical software (SAS Institute, Cary, NC) and evaluated differences between categorical variables were tested using Pearson’s Chi-square test. For differences between continuous variables, we used the Student’s t-test or Wilcoxon rank sum test as appropriate. p < 0.05 was considered statistically significant. Because this was a pilot feasibility study, no specific sample size calculation was performed.
Results
A total of 20 patients were enrolled in this study. Table 1 shows their backgrounds and characteristics. The tumor location was in the descending colon in 11 patients (55%) and in the sigmoid-descending colon junction in 9 (45%). There were 7 cases of Stage I, 1 case of Stage II, and 11 cases of Stage III, and 1 case of Stage IV disease according to the TNM classification of malignant tumors. In 3 cases (15%), self-expandable metallic stent placement was performed preoperatively for release of stenosis due to the tumor. There was no significant difference between the ICG-guided and conventional group regarding background characteristics.
Table 2 displays the surgical outcomes. Median distance from the peritoneal reflection to the tumor (PR-Tu) was 40 (range, 22–66) cm. Median distance from the peritoneal reflection to the contrast limit (PR-CL) was 40 (range, 17–66) cm. Blood flow was observed to reach up to the level of the primary tumor in 18 cases. In 2 cases blood flow did not reach up to around the tumor, but only reached up to 10 and 5 cm distal to the tumor, which corresponds to 25 and 35 cm from the peritoneal reflection, respectively. Thus, we divided the IMA at the root in all cases (100%). Median duration between appearance of the fluorescence on imaging at the aortic bifurcation to the sigmoid colon at the level of the sacral promontory was 24 (3–61) s.
Following mesenteric division of the anal side, we checked the blood flow extracorporeally using fluorescence imaging, and in 2 cases the ICG dye did not reach up to the planned DM, and so the actual DM was reset to 7 and 8 cm on the anal side of the planned DM in both cases (33 and 17 cm from PR, respectively). Among these 2 cases, the latter case was one of the above-mentioned cases in which blood flow did not reach up to the tumor. We also evaluated the color of the colon before anastomosis and no demarcation line was observed in all cases, including these 2 cases.
We performed anastomosis as FEEA in 17 cases (85%) and as DST in 3 cases, because the length (from PR to DM) was too short (15 cm) for FEEA. Postoperative complications (Clavien-Dindo ≥ 2) were observed in one patient with grade 2 surgical site infection. There were no postoperative complications associated with anastomosis, including leakage or stenosis in both groups. In one case, the specimen was removed with 26 cm of the PM because of colonic diverticulum.
Table 3 presents pathological data. The ICG-guided group had a longer proximal margin than the conventional group (p = 0.037). But there was no significant difference regarding the DM. Median number of total dissected lymph nodes was 16 (range, 5–42), that of intermediate mesocolic lymph nodes was 5 (range, 0–14), and that of principal lymph nodes was 2 (range, 0–11). The number of principal and total nodes in the IMA-ligated group was significantly greater than in the conventional IMA-preserved group. We observed lymph node metastasis in 9 of the 20 cases (45%); 3 of 9 cases of lymph node metastasis to the intermediate mesocolic lymph nodes and 1 of 9 cases of metastasis to principal lymph nodes. At a median follow up period of 15 (range, 6–22) months, no anastomotic complications such as stenosis or ischemic colitis were observed. We found liver metastases in 1 patient 7 months after surgery. As of this writing, all patients are alive with no signs of local recurrence.
Discussion
We confirmed the safety and effectiveness of ligation of the IMA for cancer of the descending colon without sacrificing additional distal colon, using fluorescence evaluation of blood flow of the remnant colon. The blood flow to the rectosigmoid from the anal side originated more proximally than expected. We believe this information is beneficial for radical surgery, including CME with CVL, especially for advanced cancer of the descending colon.
With high ligation of the IMA, blood flow to the remnant sigmoid colon is a major concern because it originates from the internal iliac artery. Several studies have reported the length of viable sigmoid colon after IMA ligation for cancer of the descending colon. According to Golighter, a reliable length of the distal sigmoid colon is about 2 inches (5.1 cm) from the PR [7]. It has been reported that blood flow might reach up to about 20 cm from the PR in a trial involving 9 patients using fluorescein and PaO2 [8] Sudeck reported a case of no anastomosis in the marginal artery between the IMA and the last branch of the SRA, and so this point is generally known as the critical point of Sudeck [9].
Clinical evaluation of blood flow in the intestine is carried out using color tone, visual recognition, palpation of the pulsating marginal blood vessel, and echocardiography. However, these are challenging in terms of objectivity and reproducibility. The effectiveness of intestinal blood flow evaluation using fluorescence imaging was recently described [10]. and Kudszus et al. were the first to report fluorescence imaging for colorectal surgery [11]. Reports also exist of the usefulness of ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis [6, 12]. ICG fluorescence imaging has several advantages over conventional methods in that it is simple and objective, it is not affected by the volume of pericolic fat, and it can be performed frequently during surgery [13,14,15].
In this study, fluorescence was observed in most cases (18/20) up to the tumor site (median 40 cm from the PR), and the median value of the distal dissection line was 30 cm from the PR in 20 cases. We determined that blood flow from the anal side may extend further than previously believed. Furthermore, because a longer segment of the distal colon was preserved, most of the anastomoses were performed with FEEA, and not DST. Anastomosis-associated complications such as stenosis or leakage might occur more frequently in DST compared with FEEA [16]. Therefore, knowing the optimal length of viable sigmoid colon before IMA ligation would be beneficial. In terms of postoperative short-term safety, our techniques were not obviously inferior to conventional IMA preservation. As for postoperative complications, only 1 case of surgical site infection (Grade II) was not associated with intestinal anastomosis. Furthermore, there were no occurrences of anastomotic leakage or stenosis.
Another concern regarding use of ICG is the time to distribution to the intestine. We found that the time until intestine imaging varied considerably. The relationship between this interval and patient clinicopathological factors such as height, weight, BMI, pulse pressure, and mean blood pressure was not significant.
To evaluate the pathological benefit of high ligation of the IMA, lymph node yield and length of the resected intestine in our study were compared with those in 20 patients who underwent SRA-preserving surgery for cancer of the descending colon over the preceding 4 years. Regardless of possible bias between groups, more lymph nodes tended to be dissected in our group, especially the total lymph nodes and principal lymph nodes (Table 3). According to the Japanese Society for Cancer of the Colon and Rectum database, the rate of intermediate and principal lymph node metastasis in primary T3/4 colon cancer was 13.6% and 3.3%, respectively [17]. Therefore, we believe it is important to clear the lymph nodes around the root of the IMA based on the principle of CME with CVL, especially for advanced cancer. This might be especially true in cancer of the descending colon, where the distance from the tumor to the IMA is shorter than that for sigmoid or rectal cancer.
Our study has several limitations. First, the small number of included patients means that we cannot draw any concrete conclusion. Furthermore, because cancer of the descending colon comprises only about 5% of all colorectal cancers, a multicenter trial with a large number of patients is needed to confirm our results. Second, ICG fluorescence imaging is not quantitative, so it is difficult to determine the cutoff point for safe blood flow [18]. Third, our study does not include long-term outcomes, such as anastomotic stricture or ischemic complications of the remnant distal colon. Fourth, we have no data demonstrating the superiority of survival data.
Despite these potential limitations, we believe that useful information can be gleaned from our investigation into surgery for cancer of the descending colon, especially with advanced tumor and suspected lymph node metastasis around the root of the IMA.
Conclusion
Our study demonstrated the safety and effectiveness of high ligation of the IMA for cancer of the descending colon without sacrificing additional distal colon using fluorescence evaluation of blood flow in the remnant colon.
References
Martling AL, Holm T, Rutqvist L, Moran BJ, Heald RJ, Cedermark B et al (2000) Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Lancet 356:93–96
Hohenberger W, Weber K, Matzel K, Papadopoulos T (2009) Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Color Dis. 11(4):354–364. https://doi.org/10.1111/j.1463-1318.2008.01735.x
Gao Z, Wang C, Cui Y, Shen Z, Jiang K, Shen D et al (2018) Efficacy and safety of complete mesocolic excision in patients with colon cancer: three-year results from a prospective, nonrandomized, double-blind controlled trial. Ann Surg 271(3):1–8
Griffiths JD (1956) Surgical Anatomy of the Blood Supply. Ann R Coll Surg Engl 19(4):241–256
Goligher JC (1954) The adequacy of the marginal blood-supply to the left colon after high ligation of the inferior mesenteric artery during excision of the rectum. Br J Surg. 41(168):351–353. https://doi.org/10.1002/bjs.18004116804
Kawada K, Hasegawa S, Wada T, Takahashi R, Hisamori S, Hida K et al (2017) Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg Endosc 31(3):1061–1069
Goligher JC (1949) The blood-supply to the sigmoid colon and rectum with reference to the technique of rectal resection with restoration of continuity. Br J Surg. 37(146):157–162. https://doi.org/10.1002/bjs.18003714604
Maeda K (1990) Experimental and Clinical Study of the Colonic Ischemic State in the Distal Colon after Left-sided Hemicolectomiy. Nippon Daicho Komonbyo Gakkai Zasshi 43:542–553
SUDECK P (1907) Munch med. Wschr 54(1314):93798
James DRC, Ris F, Yeung TM, Kraus R, Buchs NC, Mortensen NJ et al (2015) Fluorescence angiography in laparoscopic low rectal and anorectal anastomoses with pinpoint perfusion imaging- a critical appraisal with specific focus on leak risk reduction. Color Dis 17:16–21
Kudszus S, Roesel C, Schachtrupp A, Höer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck’s Arch Surg 395(8):1025–1030. https://doi.org/10.1111/j.1463-1318.2008.01735.x
Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E (2017) Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc 31(4):1836–1840
Watanabe J, Ota M, Suwa Y, Suzuki S, Suwa H, Momiyama M et al (2015) Evaluation of the intestinal blood flow near the rectosigmoid junction using the indocyanine green fluorescence method in a colorectal cancer surgery. Int J Colorectal Dis 30(3):329–335
Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA et al (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 220(1):82–92.e1. https://doi.org/10.1016/j.jamcollsurg.2014.09.015
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V et al (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259(4):700–707
Kaivahara H, Kobayashi T, Watanabe K, Shinoda T, Kashiwagi H, Yanaga K (2009) Colorectal stapling anastomosis without transanal procedure for anterior reseciton. Hepatogastroenterology 56(90):352–354
Japanese Society for Cancer of the Colon and Rectum. Multi-Institutional Registry of Large Bowel Cancer in Japan. Vol.30 Cases terated in 2003–2004.
Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S et al (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31(10):4184–4193
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Taro Munechika, Ryuji Kajitani, Yoshiko Matsumoto, Hideki Nagano, Akira Komono, Naoya Aisu, Mitsuaki Morimoto, Gumpei Yoshimatsu, Yoichiro Yoshida, and Suguru Hasegawa have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Munechika, T., Kajitani, R., Matsumoto, Y. et al. Safety and effectiveness of high ligation of the inferior mesenteric artery for cancer of the descending colon under indocyanine green fluorescence imaging: a pilot study. Surg Endosc 35, 1696–1702 (2021). https://doi.org/10.1007/s00464-020-07556-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07556-x