Abstract
Background
There has been an increase in sleeve gastrectomy (SG) procedures being performed worldwide, and a paralleled rise in prevalence of gastric sleeve stenosis (GSS). Symptoms include dysphagia, reflux, and obstructive symptoms. Upper gastrointestinal series (UGIS) is commonly performed in the diagnostic algorithm prior to referral for endoscopic dilation; however, little is known about its utility in making a diagnosis. Our aim was to evaluate positive predictive value (PPV) and negative predictive value (NPV) of UGIS in detection of GSS.
Methods
We performed a retrospective analysis of a prospectively collected database at a tertiary center for patients referred with nausea/vomiting or obstructive symptoms following SG between 2017 and 2019. All patients underwent upper endoscopy (EGD) for evaluation of GSS. Serial balloon dilations were performed for GSS with increasing balloon size and/or filling pressure until symptom resolution or referral for surgical revision. Primary outcomes were PPV and NPV for UGIS in predicting GSS. Secondary outcomes included EGD findings and symptom response to dilation.
Results
Thirty consecutive patients were included in the analyses. The most common presenting symptoms were nausea (66.7%), vomiting (60.0%) reflux (66.7%), and abdominal pain (54.8%). Twenty-two (73.3%) patients underwent UGIS prior to EGD. On diagnostic EGD, 27 (87.1%) patients were diagnosed with GSS. The sensitivity and NPV of UGIS to detect GSS was 30.0%, and 12.5%, respectively. All 6 patients with GSS on UGIS also had GSS on endoscopic evaluation (specificity = 100%, PPV = 100%). Twenty-six (86.6%) patients had resolution of symptoms with a mean 1.97 ± 1.13 dilations.
Conclusion
UGIS following SG has low NPV to evaluate for GSS. Independent of the UGIS findings, majority of patients found to have GSS on EGD had symptom improvement with dilations. The utility of UGIS is limited for diagnosing GSS and when suspicion for GSS is high, patients should be referred directly for EGD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Obesity is an ongoing problem affecting 93 million individuals in the United States with a prevalence reaching 40% [1]. Sleeve gastrectomy (SG) is a rapidly growing surgical option given its efficacy and safety profile, with lower complication rates when compared to Roux-en-Y gastric bypass (RYGB) [2,3,4] However, adverse events do occur and include gastroesophageal reflux disease, leaks along the staple line, and gastric sleeve stenosis (GSS) [5,6,7].
GSS, defined as narrowing of the sleeve or significant angulation of the gastric lumen, occurs in approximately 0.1–3.9% of patients [8,9,10,11,12]. As the number of SG procedures increase, we have seen a parallel rise in the prevalence of GSS. Typical symptoms include nausea, vomiting, epigastric abdominal pain, dysphagia, reflux and regurgitation [8, 13]. The time between operation and presentation with GSS symptoms is quite variable and can occur days or months to years after initial surgery [8, 14]. The etiology of GSS is not entirely clear but is felt to occur due to over-retraction of the greater curvature during stapling, early post-operative edema, ischemia, torsion, or scarring of the sleeve [5, 14].
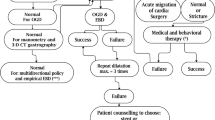
Current diagnostic algorithms have proposed radiographic evaluation with an upper gastrointestinal series (UGIS) for evaluation of these symptoms [14]. Independent of these findings, simultaneous referral for upper endoscopy is not uncommon. Little is known about the utility of performing an UGIS prior to endoscopy. The aim of the current study was to evaluate the utility of UGIS for detecting GSS prior to endoscopic evaluation. We hypothesized that UGIS would have a low sensitivity in detection of GSS and is likely an unnecessary test in evaluation of these patients.
Materials and methods
Study sample
We performed a retrospective analysis of a prospectively collected database at a single tertiary care center for adult patients referred with nausea, vomiting and/or obstructive symptoms following SG between May 2017 and May 2019. All patients in this study underwent upper endoscopy (EGD) for evaluation of GSS. Consistent with prior literature, the diagnosis of stenosis was confirmed endoscopically when the passage of the endoscope through the gastric lumen was difficult due to narrowing of the sleeve or required considerable scope rotation due to twisting or angulation [12]. Patients with simultaneous sleeve leaks were excluded from inclusion. The study was approved by the Institutional Review Board prior to inception.
Study variables
Data collected included age, sex, date of bariatric surgery, pre-surgical body mass index (BMI), nadir BMI, BMI at time of referral, symptoms, UGIS findings, procedural information, and response to treatment.
Procedure protocol
A single expert bariatric endoscopist performed all of the endoscopic procedures. A diagnostic upper endoscopy was completed in all patients to confirm the diagnosis of sleeve stenosis and obtain measurements of standard upper GI landmarks. If stenosis was demonstrated, patients were treated with balloon dilation using a 20 mm hydrostatic balloon (Cook Medical, Bloomington, IN) at the level of the gastric stenosis followed by successive pneumatic balloon dilation (PBD) (Boston Scientific, Marlborough, MA). Patients were brought back for repeat PBD every 2–4 weeks with increasing balloon size (30 mm, 35 mm, 40 mm) and/or filling pressure until resolution of symptoms was achieved or patient was referred for surgical revision. Care was taken to avoid the pylorus and gastroesophageal junction. Surgical referral was performed in patients who did not respond to endoscopic dilation.
Outcomes
Primary outcomes were PPV and NPV for UGIS in predicting GSS. Secondary outcomes included EGD findings and symptom response to dilation. Patients were followed for a minimum of 6 months after completion of the final dilation. Patient-reported symptom response was assessed by the provider at follow-up clinic visits or subsequent procedures. Symptoms were rated on a Likert scale of 1 (completely better) to 5 (no improvement, same as prior dilation). Failure was defined as the persistence of symptoms (score 4 or 5) or need for surgical revision. Success was defined as durable resolution of symptoms that allowed the patient to avoid surgery.
Statistical analyses
Descriptive statistics were summarized as proportions and presented as means and standard deviations. Fisher’s exact test for binary variables and Student’s t test or Wilcoxon signed-rank test for continuous variables were applied to assess baseline differences. Overall test sensitivity, specificity, and positive predictive value (PPV) and negative predictive value (NPV) of the UGIS test were calculated. All statistical analyses were performed using StataMP v14.1.412 (StataCorp LLC, College Station, TX).
Results
During the 2-year study period, 30 consecutive patients with SG were referred for further evaluation with EGD for symptoms of nausea (66.7%, n = 20), vomiting (60.0%, n = 18), reflux (66.7%, n = 20), dysphagia (20%, n = 6), and/or abdominal pain (54.8%, n = 17). Demographic characteristics are shown in Table 1. Mean (± SD) age was 44.3 ± 11.6 and 80.7% (n = 25) of the patients were female. Ninety percent (27/30) of patients presented with late symptoms (> 30 days) following surgery. Mean (± SD) number of days from initial gastric sleeve surgery to referral for symptoms was 994.2 ± 1549.1 (range: 16 to 8402 days). Mean (± SD) number of days between surgery and upper GI series was 696.8 ± 656.2 (range: 38 to 2281 days). Mean (± SD) number of days between surgery and endoscopy was 1198.9 ± 1487.6 (range: 39 to 8448 days). Mode bougie dilator size used for sleeve gastrectomy was 36 French (range: 32–48 French) (Table 2).
Upper GI series was performed in 73.3% (22/30) of patients prior to EGD. Of these patients, 27.3% (n = 6) were found to have GSS on upper GI series (Fig. 1A). All patients included in this study underwent diagnostic EGD based on presenting symptoms. Of these, 26/30 (86.7%) patients were noted to have endoscopic findings consistent with GSS (Fig. 1B). The sensitivity and NPV of UGIS to detect GSS were 30.0% and 12.5%, respectively. All 6 patients with GSS demonstrated on UGIS also had GSS demonstrated on endoscopic evaluation (specificity = 100%, PPV = 100%). Percent GSS detected on UGIS and EGD by Bougie dilator size is shown in Table 2.
Endoscopic pneumatic dilation was performed in all patients identified with GSS on upper endoscopy (Fig. 1C). The mean number of dilations per patient was 1.97 ± 1.13. Twenty-six (86.6%) patients had resolution of symptoms with successive dilations. Three (9.6%) patients underwent conversion to RYGB due to persistent symptoms or lack of durable resolution of symptoms. No patients were lost to follow-up during the study period (Fig. 2).
Discussion
Sleeve gastrectomy procedures have become increasingly performed in the United States over the past decade [15, 16]. GSS can be an early or late adverse event of the procedure, and its prevalence is increasing given the increasing number of procedures being performed. In this study, we found that an UGIS for detection of GSS has a low sensitivity (30%) and NPV (12.5%). UGIS was highly specific for GSS, with a specificity of 100%. Interestingly, 87.1% of patients were diagnosed with GSS on endoscopy, and nearly 90% responded to serial dilations. These results suggest that an upper GI series has limited utility in diagnosis of GSS.
With the growing number of SG procedures being performed, it is necessary to streamline the evaluation protocol for patients presumed to have GSS. There have been no studies to date to determine the most cost-effective approach in this population. It is known from several studies that routine UGIS is not cost-effective in the detection of early post-operative leaks and thus is not recommended [6, 17,18,19].
We believe that obtaining UGIS when GSS is suspected instead of direct referral to EGD could result in a delay of care for the patient. Furthermore, it is an expensive and unnecessary test that provides limited information in the management of these patients. Regardless of the findings on UGIS, all patients in the current study underwent endoscopic evaluation. The UGIS provided little information that led to change in management for these patients. Furthermore, only 27.3% of patients were found to have GSS on UGIS, thus a large proportion of patients who have clinically significant GSS may be underdiagnosed. Expedited endoscopic evaluation may be more prudent.
Our findings from this study may help streamline the algorithm for the diagnosis of GSS; however, there are a several limitations which require further exploration. First, although we used published definitions for GSS, there is no validated quantitative method for making this diagnosis. There may be inter-reader variability in the radiographic diagnosis of GSS on UGIS. Additionally, all endoscopic evaluations in this study were performed by a single trained bariatric endoscopist. We suspect that GSS may be underdiagnosed by endoscopists who may not be as familiar with the anatomy or this entity. Future development of a standardized criterion and increasing awareness of GSS would be beneficial for all endoscopists, as this is likely to be a more commonly encountered complication given the increasing prevalence of sleeve gastrectomy. Additionally, several studies have suggested a relationship between surgical technique and the development of GSS, and results are contradictory [7, 9, 20]. In the present study, surgical technique including bougie size for initial construction of the sleeve gastrectomy was variable among providers, possibly altering the risk of GSS development. Furthermore, patients were followed for a minimum of 6 months after completion of the final dilation. It is conceivable that symptoms could still recur after this time period and ultimately require repeat dilation or surgical conversion. Lastly, all patients in this study were referred for endoscopic evaluation, likely leading to selection bias. Patients who underwent UGIS for similar symptoms and had a normal study may not have ever been referred. Conversely, patients with findings consistent with GSS may have instead been referred directly for surgical evaluation, and never sent for endoscopic intervention.
One final consideration is that our cohort predominantly included patients with late symptoms (i.e., > 30 days from surgery), and our recommendations should be applied to this population. In patients who present with early symptoms (i.e., ≤ 30 days from surgery) when sleeve stenosis is classically due to edema, ischemia, or hematoma, early radiographic imaging may be preferable in guiding decision-making due to increased risk of endoscopic evaluation in the early post-operative period [21].
In conclusion, our findings suggest that UGIS following SG has low sensitivity and NPV to evaluate for GSS. Furthermore, it provides limited information to alter the management strategy of this population. Independent of the UGIS findings, the majority of patients were found to have GSS on EGD and subsequently underwent successive dilations with improvement of their symptoms. Thus, we believe that the utility of UGIS is limited for the diagnosis of GSS and patients should instead undergo an expedited referral for EGD for evaluation and treatment of GSS.
References
Hales CM, Carroll MD, Fryar CD, Ogden CL (2015) Prevalence of obesity among adults and youth: United States, 2015–2016 key findings data from the National Health and Nutrition Examination Survey
Zellmer JD, Mathiason MA, Kallies KJ, Kothari SN (2014) Is laparoscopic sleeve gastrectomy a lower risk bariatric procedure compared with laparoscopic Roux-en-Y gastric bypass? A meta-analysis. Am J Surg 208:903–910. https://doi.org/10.1016/j.amjsurg.2014.08.002
Fridman A, Moon R, Cozacov Y et al (2013) Procedure-related morbidity in bariatric surgery: a retrospective short- and mid-term follow-up of a Single Institution of the American College of Surgeons Bariatric Surgery Centers of Excellence. J Am Coll Surg 217:614–620. https://doi.org/10.1016/j.jamcollsurg.2013.05.013
Angrisani L, Santonicola A, Iovino P et al (2015) Bariatric surgery worldwide 2013. Obes Surg 25:1822–1832. https://doi.org/10.1007/s11695-015-1657-z
Lim R, Beekley A, Johnson DC, Davis KA (2018) Early and late complications of bariatric operation. Trauma Surg Acute Care Open 3:e000219. https://doi.org/10.1136/tsaco-2018-000219
Rebibo L, Hakim S, Dhahri A et al (2016) Gastric stenosis after laparoscopic sleeve gastrectomy: diagnosis and management. Obes Surg 26:995–1001. https://doi.org/10.1007/s11695-015-1883-4
Parikh A, Alley JB, Peterson RM et al (2012) Management options for symptomatic stenosis after laparoscopic vertical sleeve gastrectomy in the morbidly obese. Surg Endosc 26:738–746. https://doi.org/10.1007/s00464-011-1945-1
Burgos AM, Csendes A, Braghetto I (2013) Gastric stenosis after laparoscopic sleeve gastrectomy in morbidly obese patients. Obes Surg 23:1481–1486. https://doi.org/10.1007/s11695-013-0963-6
Cottam D, Qureshi FG, Mattar SG et al (2006) Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc 20:859–863. https://doi.org/10.1007/s00464-005-0134-5
Boza C, Salinas J, Salgado N et al (2012) Laparoscopic sleeve gastrectomy as a stand-alone procedure for morbid obesity: report of 1,000 cases and 3-year follow-up. Obes Surg 22:866–871. https://doi.org/10.1007/s11695-012-0591-6
Lacy A, Ibarzabal A, Obarzabal A et al (2010) Revisional surgery after sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech 20:351–356. https://doi.org/10.1097/SLE.0b013e3181f62895
Manos T, Nedelcu M, Cotirlet A et al (2017) How to treat stenosis after sleeve gastrectomy? Surg Obes Relat Dis 13:150–154. https://doi.org/10.1016/j.soard.2016.08.491
Binda A, Jaworski P, Tarnowski W (2013) Stenosis after sleeve gastrectomy – cause, diagnosis and management strategy. Polish J Surg 85:730–736. https://doi.org/10.2478/pjs-2013-0112
Agnihotri A, Barola S, Hill C et al (2017) An algorithmic approach to the management of gastric stenosis following laparoscopic sleeve gastrectomy. Obes Surg 27:2628–2636. https://doi.org/10.1007/s11695-017-2689-3
Young MT, Gebhart A, Phelan MJ, Nguyen NT (2015) Use and outcomes of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass: analysis of the American College of Surgeons NSQIP. J Am Coll Surg 220:880–885. https://doi.org/10.1016/j.jamcollsurg.2015.01.059
Nguyen NT, Nguyen B, Gebhart A, Hohmann S (2013) Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. J Am Coll Surg 216:252–257. https://doi.org/10.1016/j.jamcollsurg.2012.10.003
Terterov D, Leung PH-Y, Twells LK et al (2017) The usefulness and costs of routine contrast studies after laparoscopic sleeve gastrectomy for detecting staple line leaks. Can J Surg 60:335–341. https://doi.org/10.1503/CJS.015216
Brockmeyer JR, Simon TE, Jacob RK et al (2012) Upper gastrointestinal swallow study following bariatric surgery: institutional review and review of the literature. Obes Surg 22:1039–1043. https://doi.org/10.1007/s11695-012-0658-4
Mizrahi I, Tabak A, Grinbaum R et al (2014) The utility of routine postoperative upper gastrointestinal swallow studies following laparoscopic sleeve gastrectomy. Obes Surg 24:1415–1419. https://doi.org/10.1007/s11695-014-1243-9
Lalor PF, Tucker ON, Szomstein S, Rosenthal RJ (2008) Complications after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 4:33–38. https://doi.org/10.1016/j.soard.2007.08.015
Zundel N, Hernandez JD, Neto MG, Campos J (2010) Strictures after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech 20:154–158. https://doi.org/10.1097/SLE.0b013e3181e331a6
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Sean Bhalla and Jessica X. Yu: No conflict of interest. Oliver A. Varban: Blue Cross Blue Shield of Michigan: salary support for leadership and participation in quality improvement initiatives. Allison R. Schulman: Apollo Endosurgery: consultant; Boston Scientific: consultant; and MicroTech: consultant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhalla, S., Yu, J.X., Varban, O.A. et al. Upper gastrointestinal series after sleeve gastrectomy is unnecessary to evaluate for gastric sleeve stenosis. Surg Endosc 35, 631–635 (2021). https://doi.org/10.1007/s00464-020-07426-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07426-6