Abstract
Background
While multiple studies have evaluated endoscopic submucosal dissection (ESD) and transanal endoscopic microsurgery (TEM) to remove large rectal tumors, there remains a paucity of data to evaluate their comparative efficacy and safety. The primary aim of this study was to perform a structured systematic review and meta-analysis to compare efficacy and safety of ESD versus TEM for the treatment of rectal tumors.
Methods
Individualized search strategies were developed from inception through November 2018 in accordance with PRISMA guidelines. Measured outcomes included pooled enbloc resection rates, margin-negative (R0) resection rates, procedure-associated adverse events, and rates of recurrence. This was a cumulative meta-analysis performed by calculating pooled proportions. Heterogeneity was assessed with Cochran Q test and I2 statistics, and publication bias by funnel plot using Egger and Begg tests.
Results
Three studies (n = 158 patients; 55.22% male) were included in this meta-analysis. Patients with ESD compared to TEM had similar age (P = 0.090), rectal tumor size (P = 0.108), and diagnosis rate of adenoma to cancer (P = 0.53). ESD lesions were more proximal as compared to TEM (8.41 ± 3.49 vs. 5.11 ± 1.43 cm from the anal verge; P < 0.001). Procedure time and hospital stay were shorter for ESD compared to TEM [(79.78 ± 24.45 vs. 116.61 ± 19.35 min; P < 0.001) and (3.99 ± 0.32 vs. 5.83 ± 0.94 days; P < 0.001), respectively]. No significant differences between enbloc resection rates [OR 0.98 (95% CI 0.22–4.33); P = 0.98; I2 = 0.00%] and R0 resection rates [OR 1.16 (95% CI 0.36–3.76); P = 0.80; I2 = 0.00%] were noted between ESD and TEM. ESD and TEM reported similar rates of adverse events [OR 1.15 (95% CI 0.47–2.77); P = 0.80; I2 = 0.00%] and rates of recurrence [OR 0.46 (95% CI 0.07–3.14); P = 0.43; I2 = 0.00%].
Conclusion
ESD and TEM possess similar rates of resection, adverse events, and recurrence for patients with large rectal tumors; however, ESD is associated with significantly shorter procedure times and duration of hospitalization. Future studies are needed to evaluate healthcare utilization for these two strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In the United States, it is estimated there will be 44,180 new diagnoses of rectal cancer in 2019 alone [1]. Although standard treatment for advanced rectal cancer has traditionally been considered anterior resection (AR) or abdominoperineal resection (APR), less invasive alternative modalities such as endoscopic submucosal dissection (ESD) and transanal endoscopic microsurgery (TEM) have emerged as effective treatments to achieve local excision of rectal tumors with a reduced associated morbidity compared to traditional surgery. Compared with conventional surgery, ESD and TEM are less traumatic, resulting in less post-procedure pain, faster recovery, shorter hospital duration, and more rapid return to daily life [2,3,4].
While ESD is a specialized resection technique that enables endoscopic enbloc resection of colorectal lesions using a modified needle knife for submucosal dissection, TEM is a surgical procedure that employs an operative rectoscope and allows for full-thickness resection of tumors located 5 to 18 cm from the anal verge [5,6,7]. Both techniques are considered minimally invasive procedures to treat benign rectal adenomas, intramucosal cancer, and superficial submucosal cancer and have largely replaced AR and APR for these lesions. However, there are limited data available to determine if one approach is superior to the other [2, 8, 9].
As such, the primary aim of this study was to perform a structured systematic review and meta-analysis to compare efficacy and safety of ESD versus TEM for the treatment of rectal tumors.
Materials and methods
Study design and search strategy
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement outline for reporting systematic reviews and meta-analyses and was conducted following a prior established protocol [10]. Individualized searches of PubMed, EMBASE, Web of Science, and Cochrane databases were performed from inception through November 30, 2018. The following medical subject heading (MESH) terms included: rectal tumor or rectal cancer. For articles related to rectal tumor, subject heading search terms and title and abstract were reviewed for endoscopic surgery, endoscopic removal, endoscopic submucosal dissection (ESD), and transanal endoscopic microsurgery (TEM).
All relevant articles irrespective of year of publication, type of publication, or publication status were included. The titles and abstracts of all potentially relevant studies were screened for eligibility. The reference lists of studies of interest were then manually reviewed for additional articles by cross checking bibliographies. Two reviewers (TRM and ANB) independently screened the titles and abstracts of all the articles according to predefined inclusion and exclusion criteria. Any differences were resolved by mutual agreement and in consultation with the third reviewer (KEH). In the case of studies with incomplete information, contact was attempted with the principal authors to obtain additional data. Institutional IRB approval and written consent was not required given the design of this systematic review and meta-analysis.
Study selection criteria
Randomized controlled trials, observational studies, and case series evaluating ESD and TEM were included in this analysis. Studies were included if patients were adults ≥ 18 years of age, had a diagnosis of rectal tumor, and underwent ESD and TEM procedures for tumor removal. Included studies were required to be directly comparative studies including both removal techniques. Alterative tumor removal procedures such as endoscopic mucosal resection (EMR) were not included. Mandatory outcomes to merit study inclusion were pooled enbloc resection rates, margin-negative (R0) resection rates, procedure-associated adverse events, or rates of recurrence. Only human subject studies were considered for this meta-analysis. Multiple published works from similar authors were evaluated for overlapping enrollment times to preserve independence of observations. A study was excluded if deemed to have insufficient data, as were review articles, editorials, and correspondence letters that did not report independent data. Case series and reported studies with < 5 patients were excluded in effort to limit selection bias.
Outcome measures
The primary outcome was a comparative review of ESD versus TEM with regard to pooled enbloc resection rates, margin-negative (R0) resection rates, procedure-associated adverse events, and rates of recurrence. Secondary outcomes included baseline patient and procedure characteristics including rectal tumor size and location, diagnosis rate (defined as adenoma to cancer rate), duration of procedure (min), and mean duration of hospitalization.
Risk of bias and quality assessment
Risk of bias was assessed using the Cochrane Collaboration’s risk of bias in non-randomized studies of interventions (ROBINS-I) tool for observational studies [11]. In this meta-analysis, publications were deemed low risk of bias if ≥ 50% of the above domains were judged as low risk. The quality of observational studies was evaluated using the Newcastle–Ottawa Quality Assessment Scale [12]. Two authors (TRM and ANB) independently extracted data and assessed the risk of bias and study quality for each of the articles. Any disagreements were resolved by discussion and consensus, and in consultation with the third reviewer (KEH).
Investigations of heterogeneity
Heterogeneity was assessed for the individual meta-analyses using the Chi-squared test and the I2 statistic [13]. Significant heterogeneity was defined as P < 0.05 using the Chi-squared or I2 > 50%. A random effects model was used except for when statistical heterogeneity was not significant. Differences in subgroups were assessed using a Chi-squared test for interaction with a P < 0.05 defined as statistically significant. To assess for publication bias, a funnel plot was created and visually inspected for asymmetry [14].
Statistical analysis
This meta-analysis was performed by calculating pooled proportions. After appropriate studies were identified through systematic review, the individual study proportion was transformed into a quantity using the Freeman–Tukey variant of the arcsine square root transformed proportion. Then the pooled proportion was calculated as the back transform of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed effects model and DerSimonian–Laird weights for the random effect model [15, 16]. All weighted pool rates involved 95% confidence intervals and were analyzed using fixed or random effects models based on the heterogeneity of the sample. Tabular and graphical displays were performed in Review Manager 5 (RevMan 5.3). Combined weighted proportions and additional analyses were determined by the use of the Stata 13.0 software package (Stata Corp LP, College Station, TX).
Results
Characteristics of included studies
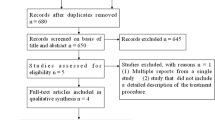
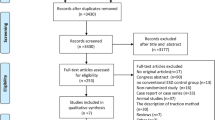
This systematic review and meta-analysis included a total of 3 studies (n = 158) [2, 8, 9]. A PRISMA flow chart of search results is shown in Fig. 1. All included studies were retrospective cohort studies with no prospective or randomized control trials found via the above search criteria. One study by Jung et al. separated cohorts based upon epithelial and subepithelial lesions [9]. Mean age of included patients was 61.94 ± 4.84 years with 55.22% males. Average study follow-up of both ESD and TEM was 15.87 ± 9.45 months. Mean tumor rectal size overall was 31.33 ± 11.17 mm at a mean distance of 6.67 ± 3.08 cm from the anal verge. Additional study characteristics are highlighted in Table 1.
ESD versus TEM
Among included comparative studies, mean age of patients in the ESD cohort was similar to patients who underwent TEM (62.52 ± 4.91 vs. 61.20 ± 4.69 years; P = 0.090). Mean follow-up duration for TEM was almost double that of ESD (20.47 ± 10.33 vs. 12.31 ± 6.88 months; P < 0.001). With regard to ESD compared to TEM, the size of rectal tumor was similar between two groups (32.58 ± 13.29 vs. 29.70 ± 7.41 mm; P = 0.108), respectively. Location of ESD lesions was more proximal as compared to TEM (8.41 ± 3.49 vs. 5.11 ± 1.43 cm from the anal verge; P < 0.001).
With regard to primary outcome measures, there were no significant differences between enbloc resection rates [OR 0.98 (95% CI 0.22 to 4.33); P = 0.98; I2 = 0.00%] and R0 resection rates [OR 1.16 (95% CI 0.36 to 3.76); P = 0.80; I2 = 0.00%] between ESD and TEM groups—Figs. 2 and 3. Rate of adenoma to cancer diagnosis was similar between the ESD and TEM groups as well [OR 0.80 (95% CI 0.41 to 1.58); P = 0.53; I2 = 80.00%]. ESD and TEM also reported similar rates of procedure-associated adverse events [OR 1.15 (95% CI 0.47 to 2.77); P = 0.80; I2 = 0.00%] and rates of tumor recurrence [OR 0.46 (95% CI 0.07 to 3.14); P = 0.43; I2 = 0.00%]—Figs. 4 and 5. Cumulative pooled rates for ESD and TEM are shown in Table 2. Despite similar primary outcome measures, procedure time and hospital stay were significantly shorter for ESD as compared to TEM [(79.78 ± 24.45 vs. 116.61 ± 19.35 min; P < 0.001) and (3.99 ± 0.32 vs. 5.83 ± 0.94 days; P < 0.001), respectively].
Risk of bias assessment
All studies were assessed using ROBINS-I, and the Newcastle–Ottawa Quality Assessment Scale with authors’ judgements about each risk of bias item for all included studies is highlighted in Fig. 6A. A risk of bias summary graph is also available in Fig. 6B. Testing for publication bias with funnel plot asymmetry was not performed given the limited number of included studies.
Discussion
Overall, the results of this systematic review and meta-analysis demonstrate that ESD and TEM are similar in efficacy and safety for patients with large rectal lesions. Pooled enbloc resection rate and R0 resection rate for ESD and TEM were comparable [(97% versus 97%) and (94% versus 93%), respectively]. Adverse event rate and rate of recurrence for ESD versus TEM was also similar [(21% versus 18%) and (1% versus 3%), respectively]. Secondary aims of this study highlight that ESD resulted in decreased procedure time as compared to TEM with shorter duration of hospital stay. Although both modalities provide effective and safe alternatives to traditional radical surgery, the technique and indication to achieve these results is different. Due to use of a rigid rectoscope, TEM is typically limited to lesions 5 cm proximal to the anal verge, while ESD relies upon a standard endoscope allowing for forward-view or retroflexed resection of lesions near the anus or at the dentate line [7, 17, 18]. The ability to treat more distal lesions provides added benefit for ESD as compared to TEM, despite comparable efficacy and safety.
A previous systematic review of non-comparator studies including 21 articles and 2077 patients revealed a significantly lower enbloc resection rate and R0 resection rate for ESD as compared to TEM [87.8% (95% CI 84.3 to 90.6) versus 98.7% (95% CI 97.4 to 99.3) P < 0.001] and [74.6% (95% CI 70.4 to 78.4) versus 88.5% (95% CI 85.9 to 90.6) P < 0.001], respectively [19]. The post-procedure adverse rate was similar between the two procedures; however, the recurrence rate for ESD was better than TEM despite lower resection rates as above [2.6% (95% CI 1.3 to 5.2) versus 5.2% (95% CI 4.0 to 6.9); P < 0.001]. While these results contradict our current meta-analysis, it is important to note these were non-comparator observational studies and all published prior to 2010—with inherent bias and inability to reflect current ESD practices, technique, and procedure evolution [9]. All studies in our analysis span from 2012 to 2018, which may account for improved proficiency of ESD practitioners, development of novel endoscopic devices for safe and accurate resections, and use of high-definition endoscopes [9, 20,21,22].
Another important consideration with regard to a preferred procedure is cost. The TEM procedure, characterized by full-thickness resection, requires the procedure to be performed under either general or spinal anesthesia, whereas ESD may be performed with conscious or deep sedation in the endoscopy unit. TEM additionally requires expensive surgical instruments which make the procedure a more expensive modality compared to ESD, even despite being an older modality [23]. A previous cost comparison study by Nam and colleagues aimed to compare costs associated with TEM versus ESD, finding median total hospital costs were significantly lower in the ESD than in the TEM group ($1214 versus $1686; P < 0.001) [4]. This is an important realization and may explain our findings of TEM being associated with a longer duration of hospitalization.
Limitations to this present study include the inherent heterogeneity bias of pooled systematic reviews and meta-analyses. Although this was evaluated with I2 and appeared to be minimal in this analysis, we cannot rule out the risk of inherent study bias, specific differences in patient population, and inter-operator variability in procedure outcomes. For this reason, published data may not fully reflect current practice and endoscopic or surgical expertise. For example, some patients undergoing TEM may be discharged from the post-anesthesia care unit after the procedure similar to patients in the endoscopy suite post-ESD. Furthermore, studies of TEM had a significantly longer follow-up period—almost double that of ESD studies, potentially allowing for more adverse events to occur. Procedure time was also not standardized among studies. The quality of included studies is also limited as no randomized controlled studies were included in this analysis, with 3 small, single-center retrospective studies included. Publication bias was also not assessed with funnel plot asymmetry as typically a minimum of 8–10 studies should be included in the meta-analysis [24].
An additional limitation relates to location of rectal lesions. Although TEM is typically recommended for tumors located 5 to 18 cm from the anal verge, the study by Kawaguti et al. reported a mean distance of 2.85 cm for lesions treated with TEM [8]. Although many surgeons perform TEM for lesions as low as the dentate line (i.e., not limiting use to lesions proximal to 5 cm from the anal verge), risk of selective bias for this study was significant. Subgroup analysis excluding this study was not possible due to limited number of studies. Ideally, it would be highly relevant to stratify our results based upon adenoma and cancer findings on pathology; however, this was not possible due to limited data and reporting style. This is very pivotal as full-thickness excision TEM can resect some T2 rectal cancers while ESD does not. It is also important to understand a large limitation of this study relates to generalizability. There is a significant learning curve or clinical expertise needed to perform an effective ESD or TEM procedure, with some institutions perhaps more adept at performing one procedure over the other—especially with regard to centers with less familiarity or proven expertise. This may result in large differences, including margin status, enbloc resection, recurrence, and other outcomes [25].
Despite these limitations, this study has several strengths. While there remains a paucity of literature, this structured systematic review and meta-analysis methodologically summarizes all available data to evaluate the comparative effectiveness of ESD versus TEM for the treatment of localized rectal tumors. Through inclusion of direct comparator studies, we also aimed to minimize selection bias. One study by Hitzler et al. appeared to be a comparator study upon initial review; however, the study was excluded as German patients undergoing TEM were compared to a literature review of ESD outcomes among Japanese patients [26]. Therefore, in effort to limit potential selection bias, this study was excluded from our meta-analysis. In addition to technical measures such as en bloc resection rates, R0 resection rates, adverse events, and rates of recurrence, we also aimed to assess surrogates of cost-effectiveness including procedure-associated times and length of hospital stay.
In conclusion, ESD and TEM appear to possess similar rates of resection, adverse events, and rate of recurrence for patients with rectal tumors. Although both have comparable efficacy and safety, ESD is associated with significantly shorter procedure times and duration of hospitalization. Future studies are needed to evaluate healthcare utilization for these two strategies and determine what subset of patients may respond better to ESD or TEM.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34
Park SU, Min YW, Shin JU et al (2012) Endoscopic submucosal dissection or transanal endoscopic microsurgery for nonpolypoid rectal high grade dysplasia and submucosa-invading rectal cancer. Endoscopy 44:1031–1036
Park HW, Byeon JS, Park YS et al (2010) Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointest Endosc 72:143–149
Nam MJ, Sohn DK, Hong CW et al (2015) Cost comparison between endoscopic submucosal dissection and transanal endoscopic microsurgery for the treatment of rectal tumors. Ann Surg Treat Res 89:202–207
Repici A, Hassan C, De Paula Pessoa D et al (2012) Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy 44:137–150
Guerrieri M, Baldarelli M, de Sanctis A, Campagnacci R, Rimini M, Lezoche E (2010) Treatment of rectal adenomas by transanal endoscopic microsurgery: 15 years’ experience. Surg Endosc 24:445–449
Kunitake H, Abbas MA (2012) Transanal endoscopic microsurgery for rectal tumors: a review. Perm J 16:45–50
Kawaguti FS, Nahas CS, Marques CF et al (2014) Endoscopic submucosal dissection versus transanal endoscopic microsurgery for the treatment of early rectal cancer. Surg Endosc 28:1173–1179
Jung Y, Lee J, Cho JY et al (2018) Comparison of efficacy and safety between endoscopic submucosal dissection and transanal endoscopic microsurgery for the treatment of rectal tumor. Saudi J Gastroenterol 24:115–121
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Sterne JA, Hernan MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Wells G, Shea B et al (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. In: 3rd symposium on systematic reviews: beyond the basics; July 3–5; Oxford. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 18 Dec 2018
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Stuart A, Ord JK (1994) Kendall’s advanced theory of statistics, 6th edn. Edward Arnold, London
Suzuki H, Furukawa K, Kan H et al (2005) The role of transanal endoscopic microsurgery for rectal tumors. J Nippon Med Sch 72:278–284
Tanaka S, Toyonaga T, Morita Y et al (2016) Feasibility and safety of endoscopic submucosal dissection for lower rectal tumors with hemorrhoids. World J Gastroenterol 22:6268–6275
Arezzo A, Passera R, Saito Y et al (2014) Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc 28:427–438
Saito Y, Sakamoto T, Nakajima T, Matsuda T (2014) Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 24:245–255
Takeuchi Y, Uedo N, Ishihara R et al (2010) Efficacy of an endo-knife with a water-jet function (Flushknife) for endoscopic submucosal dissection of superficial colorectal neoplasms. Am J Gastroenterol 105:314–322
Toyoizumi H, Kaise M, Arakawa H et al (2009) Ultrathin endoscopy versus high-resolution endoscopy for diagnosing superficial gastric neoplasia. Gastrointest Endosc 70:240–245
Maslekar S, Pillinger SH, Sharma A, Taylor A, Monson JR (2007) Cost analysis of transanal endoscopic microsurgery for rectal tumours. Colorectal Dis 9:229–234
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. http://handbook-5-1.cochrane.org. Accessed 2 Dec 2018
Hon SS, Ng SS, Chiu PW et al (2011) Endoscopic submucosal dissection versus local excision for early rectal neoplasms: a comparative study. Surg Endosc 25:3923–3927
Hitzler MH, Heintz A (2015) Single centre study: results of transanal endoscopic microsurgery of rectal tumors since 2003 vs. results of endoscopic submucosal dissection reported in the literature. Zentralbl Chir 140:645–650
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Chris C. Thompson is a consultant for Boston Scientific, Olympus America, and Apollo Endosurgery. Hiroyuki Aihara is a consultant for Olympus America, Boston Scientific, and Fujifilm Medical Systems. Thomas R. McCarty, Ahmad Najdat Bazarbashi, and Kelly E. Hathorn have no conflicts to disclose.
Ethical approval
Institutional IRB approval and written consent was not required given the design of this systematic review and meta-analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McCarty, T.R., Bazarbashi, A.N., Hathorn, K.E. et al. Endoscopic submucosal dissection (ESD) versus transanal endoscopic microsurgery (TEM) for treatment of rectal tumors: a comparative systematic review and meta-analysis. Surg Endosc 34, 1688–1695 (2020). https://doi.org/10.1007/s00464-019-06945-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06945-1