Abstract
Background
For almost 30 years, transanal endoscopic microsurgery (TEM) has been the mainstay treatment for large rectal lesions. With the advent of endoscopic submucosal dissection (ESD), flexible endoscopy has aimed at en bloc R0 resection of superficial lesions of the digestive tract. This systematic review and meta-analysis compared the safety and effectiveness of ESD and full-thickness rectal wall excision by TEM in the treatment of large nonpedunculated rectal lesions preoperatively assessed as noninvasive.
Methods
A systematic review of the literature published between 1984 and 2010 was conducted (Registration no. CRD42012001882). Data were integrated with those from the original databases requested from the study authors when needed. Pooled estimates of the proportions of patients with en bloc R0 resection, complications, recurrence, and need for further treatment in the ESD and TEM series were compared using random-effects single-arm meta-analysis.
Results
This review included 11 ESD and 10 TEM series (2,077 patients). The en bloc resection rate was 87.8 % (95 % confidence interval [CI] 84.3–90.6) for the ESD patients versus 98.7 % (95 % CI 97.4–99.3 %) for the TEM patients (P < 0.001). The R0 resection rate was 74.6 % (95 % CI 70.4–78.4 %) for the ESD patients versus 88.5 % (95 % CI 85.9–90.6 %) for the TEM patients (P < 0.001). The postoperative complications rate was 8.0 % (95 %, CI 5.4–11.8 %) for the ESD patients versus 8.4 % (95 % CI 5.2–13.4 %) for the TEM patients (P = 0.874). The recurrence rate was 2.6 % (95 % CI 1.3–5.2 %) for the ESD patients versus 5.2 % (95 % CI 4.0–6.9 %) for the TEM patients (P < 0.001). Nevertheless, the rate for the overall need of further abdominal treatment, defined as any type of surgery performed through an abdominal access, including both complications and pathology indications, was 8.4 % (95 % CI 4.9–13.9 %) for the ESD patients versus 1.8 % (95 % CI 0.8–3.7 %) for the TEM patients (P < 0.001).
Conclusions
The ESD procedure appears to be a safe technique, but TEM achieves a higher R0 resection rate when performed in full-thickness fashion, significantly reducing the need for further abdominal treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
For nearly 30 years, transanal endoscopic microsurgery (TEM) has been the optimal mainstay treatment for large rectal lesions. Initially conceived for treating benign lesions, its indications were extended to early rectal cancer treatment when Hermanek and Gall [1] assessed criteria to determine lesions at “low risk” for recurrence. One increasingly recognized advantage of the technique versus standard transanal surgery is the high rate of en bloc resection with disease-free margins, which is strictly related to the risk of recurrence [2].
With the advent of endoscopic submucosal dissection (ESD) about 10 years ago, flexible endoscopy permitted a surgical-like technique for en bloc resection of superficial lesions of the digestive tract. First indicated for the upper gastrointestinal tract [3], ESD then was applied to the lower gastrointestinal tract with promising results [4]. Although ESD represents an alternative to endoscopic mucosal resection (EMR) of the colon, its application to the rectum can be compared with TEM, both aiming to achieve en bloc R0 excision.
This study aimed to evaluate in a systematic review and meta-analysis whether ESD has clinically relevant short-term advantages in terms of safety and effectiveness compared with TEM in the treatment of large nonpedunculated rectal lesions preoperatively assessed as noninvasive.
Methods
The methods for the analysis and generation of inclusion criteria were based on the Cochrane Collaboration guidelines [5] and the PRISMA recommendations [6]. According to population, interventions, comparators, outcome measures, and setting (PICOS) criteria, patients were included if they had large nonpedunculated rectal lesions preoperatively assessed as noninvasive for which either TEM or ESD was indicated. The study methods were documented in a protocol registered and accessible at http://www.crd.york.ac.uk/prospero/ (Registration no. CRD42012001882).
Criteria for identifying studies and eligibility
The study aimed to include randomized or quasi-randomized studies that directly compared TEM and ESD. Because we knew and verified that similar studies were not available, we included prospective series that examined one of the two treatments provided they had the same inclusion and exclusion criteria. To be eligible, studies had to include reports on patients with a large (>2 cm) nonpedunculated rectal lesion preoperatively assessed as noninvasive by digital examination and/or endoscopic ultrasound (EUS) (confined to the mucosal layer) or lesions treated endoscopically by the ability to be lifted when the submucosal layer was injected below the lesion.
The exclusion criteria ruled out preoperative biopsies positive for invasive malignancy when available, TEM performed in a non-full-thickness fashion, and the impossibility to hive-off data from mixed series. Also excluded were studies reporting data on colon and rectal lesions that could not be broken up.
The criteria required that TEM had been performed in full-thickness fashion according to the technique described by Buess et al. [7]. When the technique was not specified, the authors were contacted for confirmation. Articles were included if a submucosal dissection was performed by TEM only for those lesions at risk for peritoneal opening. The criteria required that ESD had been performed after submucosal injection and lifting by any of the techniques described in the literature, including the different knives available.
Because most of the ESD series merged data on colonic and rectal lesions in a way that the two types could not be distinguished, the authors were contacted to provide a database of their published series restricted to rectal lesions only. Rectal lesions were defined as any lesion with an upper margin located within 18 cm of the anal verge, which was assessed by means of rigid rectoscopy in the TEM series and by flexible endoscopy in the ESD series.
End points
The primary end point of this review was effectiveness of resection (i.e., en bloc resection rate, defined as the rate of lesions excised in a single specimen, and R0 resection rate, defined as the rate of lesions excised with margins free of disease) as assessed by the pathologist. The secondary end points were size of the lesions excised, time for completion of the procedure, safety (i.e., postprocedural complications such as bleeding and perforation and the need for abdominal surgery to manage complications), recurrence rate as assessed by a minimum of 6 months follow-up evaluation, the need for abdominal surgery for oncologic reasons, and finally the overall need for abdominal surgery. Abdominal surgery was defined as any type of surgery performed through an abdominal access.
Search strategy
Searches of the published literature were conducted for the period between January 1984 and December 2010. Only articles published in English or German were included. Studies were identified by electronic searches of Pubmed and EMBASE.
The following strategy was used to search both PubMed and EMBASE at a single time during January 2011: endoscopic AND submucosal AND resection* OR (endoscopic AND submucosal AND dissection*) OR (endoscopic AND submucosal AND excision*) OR (endoscopic AND mucosal AND resection*) OR (endoscopic AND resection*) OR (endoscopic AND excision*) OR (endoscopic AND mucosal AND excision*) OR (endoscopic AND treatment*) OR (endoscopic AND therapy*) OR (rectoscopic AND mucosal AND resection*) OR (rectoscopic AND resection*) OR (rectoscopic AND excision*) OR (rectoscopic AND mucosal AND excision*) OR (rectoscopic AND treatment*) OR (rectoscopic AND therapy*) OR (colonoscopic AND mucosal AND excision*) OR (colonoscopic AND resection*) OR (colonoscopic AND excision*) OR (colonoscopic AND treatment*) OR (colonoscopic AND therapy*) AND (colorectal AND ‘neoplasms’/exp OR (colorectal AND tumor*) OR (colorectal AND tumour*) OR (colorectal AND neoplasm*) OR (‘rectal’/exp AND neoplasm*) OR (‘adenoma’/exp AND (‘rectum’/exp OR ‘rectal’/exp OR colorectal))) OR (tem OR (transanal AND endoscopic AND ‘microsurgery’/exp) AND ‘surgery’/exp OR transanal OR peranal AND (colorectal AND ‘neoplasms’/exp OR (colorectal AND tumor*) OR (colorectal AND tumour*) OR (colorectal AND neoplasm*) OR (‘rectal’/exp AND neoplasm*) OR (‘adenoma’/exp AND (‘rectum’/exp OR ‘rectal’/exp OR colorectal)))) AND ‘rectal’/exp AND ‘neoplasm’/exp AND (‘endoscopy’/exp OR endoscopic OR ‘microsurgery’/exp OR transanal OR mucosal OR ‘resection’/exp) OR (endoscopic AND mucosal AND ‘resection’/exp) OR (endoscopic AND submucosal AND ‘dissection’/exp) AND [1984-2010]/py.
Study selection
Titles were screened by two authors (A.A. and M.V.) to exclude nonrelated publications. Studies were excluded if the interventions, as reported in the abstracts, clearly differed from ESD or TEM or did not focus on the colorectal area.
The full text of the remaining articles was read to determine whether they were eligible for inclusion in the review. Studies were excluded in which preoperatively assessed rectal cancers were treated. When the same data of a single research group were reported in multiple publications, only the study reporting on the largest cohort was included.
Data extraction was independently performed by the two reviewers using predefined data extraction forms. A third investigator (M.M.) arbitrated in the event that agreement was not reached.
From each report, the reviewers independently collected the following data when available: year of publication, prospective or retrospective study design, enrollment period, number of patients included, mean age, gender distribution, lesion location (colon/rectum), Kudo pit-pattern classification [8], EUS, type of device used, mean operating time, mean tumor size, complication rate, rate of surgery due to complications, histology (adenoma, carcinoma in situ, invasive cancer, carcinoid), rate of histologically verified en bloc resection, rate of histologically verified complete resection (R0), rate of surgery for oncologic reasons, follow-up evaluation, histologically demonstrated recurrence, and need of further treatment for disease recurrence.
Quality assessment
All the studies fulfilling the selection criteria for this review were assessed to determine methodologic quality and risk of bias. The following quality items were scored: study design, sequence generation, cohort size, lesion type before intervention, lesion size, incidence of invasive carcinomas at final histology, length of the follow-up period, and objective definition of outcome parameters (complications and recurrence).
Table 1 reports the individual scores of quality assessment items per study. Because the data on colonic and rectal lesions from most of the ESD series were merged in such a way that they could not be distinguished, the authors were asked to provide a database of their published series restricted to rectal lesions only.
Statistical analysis
All analyses were performed according to the original treatment allocation (intention-to-treat analysis). Fixed- and random-effects meta-analyses of studies reporting single proportions were used to calculate an overall proportion. Because all the studies reported the results of only one technique in a series of patients, the logit transformed proportion of patients with recurrence or complication was used as the outcome parameter in the meta-analysis. We added 0.5 to all the cell frequencies of studies with a zero cell count.
Particularly, the random-effects model incorporates any remaining variability beyond chance that exists among studies, taking into account differences in sample size whereby proportions have been measured in each trial. This within-study variation was accounted for by using the exact binomial distribution. Individual and pooled estimates of these proportions together with 95 % confidence intervals (95 % CI) on recurrence and complication rates then were presented in the forest plots.
Operating time and tumor size were compared using their reported means and standard deviations (SDs). When means and/or SDs were not reported, they were estimated from the reported medians and ranges using the Hozo et al. [9] approach.
Potential sources of heterogeneity were explored in three different sensitivity analyses: fixed versus random-effects models (with the second model incorporating heterogeneity), cumulative meta-analysis (sequential inclusion of studies by date of publication), and influence meta-analysis (calculation of pooled estimates with omission of one study at a time).
All analyses were performed using R 2.15.0 and Meta-analyst 3.13 (for continuous outcomes) (R Foundation for Statistical Computing, Vienna, Austria) [10].
Results
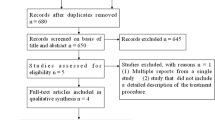
The search retrieved 9,315 studies. The selection procedure is illustrated in Fig. 1. Of the 9,315 studies, 57 were excluded because it was unclear whether full-thickness TEM procedures were performed and whether an ESD procedure was performed to treat colonic or rectal lesions. We were unable to clarify these doubts because we received no reply to our request from the respective study authors. In all, 21 studies met the inclusion criteria for a total of 2,077 patients: 11 ESD series [11–21] totaling 536 patients, and 10 TEM series [2, 22–30], totaling 1,541 patients.
The mean polyp size was 35 mm (95 % CI 31–39 mm) in the ESD series versus 40 mm (95 % CI 29–51 mm) in the TEM series (P = 0.393). The operating time was 96 min (95 % CI 84–107 min) in the ESD series versus 67 min (95 % CI 53–82 min) in the TEM series (P = 0.003).
En bloc and RO resection
The en bloc resection rate was available for 9 ESD and 9 TEM series. The pooled estimate of the proportion of patients was 87.8 % (95 % CI 84.3–90.6 %) in the ESD series and 98.7 % (95 % CI 97.4–99.3 %) in the TEM series (P < 0.001, Fig. 2). Heterogeneity was greater in the ESD series (I 2 = 60.1 %) than in the TEM series (I 2 = 46.4 %).
The cumulative meta-analysis of all 18 studies showed a progressive increase from 81.4 to 95.1 % in the proportion of patients undergoing en bloc resection. The same proportion was quite constant (94.3–95.8 %), with no study strongly affecting the results in the influential, leave-one-out meta-analysis.
The R0 resection rate was available for 9 ESD and 8 TEM series. The pooled estimate of the proportion of patients was 74.6 % (95 % CI 70.4–78.4 %) in the ESD series and 88.5 % (95 % CI 85.9–90.6 %) in the TEM series (P < 0.001, Fig. 3). Heterogeneity was lower in the ESD series (I 2 = 52.9 %) than in the TEM series (I 2 = 69.1 %). The cumulative meta-analysis of all 17 studies showed a progressive increase from 62.9 to 82.7 % in the proportion of patients undergoing R0 resection. Again, the same proportion was quite constant (81.4–83.7 %) in the influential meta-analysis.
Perioperative complications
Data regarding perioperative complications were retrieved for all 11 ESD series and 8 of the TEM series. Altogether, 1,887 patients (536 ESD and 1,351 TEM patients) were included in the analysis of complications. The complications after ESD were rectal bleeding (n = 19) and perforation (n = 20). The complications after TEM were suture leakage (n = 43), rectal bleeding (n = 30), fistulas (n = 7), urinary infection or retention (n = 6), and others (n = 11).
The proportion of patients with complications was 8.0 % (95 % CI 5.4–11.8 %) after ESD versus 8.4 % (95 % CI 5.2–13.4 %) after TEM (P = 0.874, Fig. 4). Heterogeneity was low in the ESD series (I 2 = 25.0 %) but extreme by comparison in the TEM series (I 2 = 80.5 %). A cumulative meta-analysis of all 19 studies showed a progressive increase from 4.2 to 8.6 % in the proportion of patients with complications. This proportion ranged from 7.1 to 8.7 %, without any single-trial effect, in the influential meta-analysis.
The pooled proportion of patients with perioperative events requiring additional abdominal surgery for complication control was 1.3 % (95 % CI 0.5–3.3 %) in the ESD series and 1.6 % (95 % CI 1.0–2.6 %) in the TEM series (P = 0.665, Fig. 5). Heterogeneity was absent in the ESD series (I 2 = 0.0 %) and low in the TEM series (I 2 = 14.4 %). A cumulative meta-analysis showed that 1.1–2.1 % of the patients required additional abdominal surgery. The influential meta-analysis showed a range of 1.3–1.7 %.
Histology
Only nine ESD and eight TEM series provided histology data. In all, 1,929 patients (488 ESD and 1,441 TEM patients) were included in the analyses of histology. Final pathology demonstrated an adenoma in 156 ESD patients (31.9 %) and 1,278 TEM patients (89.1 %), pTis or pT1sm1 cancers in 279 ESD patients (57.1 %) and 79 TEM patients (5.5 %), and invasive adenocarcinoma (pT1sm2 or more) in 45 ESD patients (9.2 %) and 73 TEM patients (5.1 %). Eight patients in the ESD group and four in the TEM group had another diagnosis.
The pooled estimate of the proportion of patients with invasive adenocarcinoma was 9.5 % (95 % CI 5.7–15.5 %) in the ESD series and 3.9 % (95 % CI 1.5–9.7 %) in the TEM series (P = 0.095). Heterogeneity was moderate in the ESD series (I 2 = 50.7 %) but extreme in the TEM series (I 2 = 88.2 %). The cumulative meta-analysis showed that 6.7–11.5 % of the patients required additional abdominal surgery. The influential meta-analysis showed a range of 5.0–7.8 %.
Recurrences and oncologic criteria
Only seven ESD series and nine TEM series provided recurrence data. All the ESD series reported a follow-up period of 6–12 months, whereas the TEM series reported an average follow-up period of 58.9 months (range, 1–204 months). In all, 1,811 patients (404 ESD and 1407 TEM patients) were included in the analyses of recurrences. The pooled estimate of the proportion of patients with adenoma recurrence was 2.6 % (95 % CI 1.3–5.2 %) in the ESD series and 5.2 % (95 % CI 4.0–6.9 %) in the TEM series (P = 0.068).
Heterogeneity was absent in the ESD series (I 2 = 0.0 %) and low in the TEM series (I 2 = 21.5 %). The pooled proportion of patients with perioperative events requiring additional abdominal surgery for oncologic indications or recurrence was 8.4 % (95 % CI 4.9–13.9 %) in the ESD series and 2.9 % (95 % CI 1.5–5.4 %) in the TEM series (P = 0.011). Heterogeneity was moderate in the ESD series (I 2 = 40.2 %) and greater in the TEM series (I 2 = 63.3 %).
Need for abdominal surgery
Data regarding the overall need for abdominal surgery, defined as any type of surgery performed through an abdominal access, were retrieved for eight ESD and nine TEM series. This included treatment of complications, recurrence, or major surgery for oncologic curative resection, as reported earlier. In all, 1,862 patients (455 ESD and 1407 TEM patients) were included in the analysis. The pooled estimate of the proportion of patients requiring abdominal surgery was 8.4 % (95 % CI 4.9–13.9 %) in the ESD series and 1.8 % (95 % CI 0.8–3.7 %) in the TEM series (P < 0.001, Fig. 6). Heterogeneity was moderate in both the ESD (I 2 = 40.2 %) and TEM (I 2 = 48.1 %) series.
Discussion
One of the most important risk factors for recurrence of rectal lesions is an R1 resection [2, 31, 32], which is obviously less probable when an en bloc resection is attempted. A recent systematic review by Barendse et al. [33] reported a recurrence rate of 11.2 % at 3 months after piecemeal EMR for colorectal lesions, which dropped to 1.5 % at 3 months after further endoscopic treatment. The authors claimed that this demonstrated the equivalence of EMR and TEM. However, the analysis contained a number of flaws. The two major flaws were that (1) all but one endoscopic series included only benign lesions, which suggested an evident selection of cases based on postoperative histology, and that (2) most of the TEM series included cases managed by a partial wall excision rather than a full-thickness technique, as suggested by most expert authors [29].
Due to the high rate of preoperatively misdiagnosed malignancies, piecemeal resection, as obtained by EMR, should not be performed when valid alternatives are available. Currently, surgeons performing endoscopic resection of a noninvasive rectal lesion should aim to use an ESD technique. Although rectal lesions currently are diagnosed earlier than in the past and can be treated with a variety of different techniques, we found no randomized or quasi-randomized study comparing ESD with TEM. Furthermore, although a meta-analysis of only randomized controlled trials would be ideal, case series data are the only evidence available to date.
The major limitation in the meta-analysis of the aforementioned data was the potential confounding by a systematic difference in patient characteristics between the two groups. In fact, although patients eligible for ESD will necessarily be assessed as having a superficial lesion, TEM often is performed also for those with an invasive lesion and almost always as a full-thickness excision. For this reason, we defined strict inclusion criteria that required a rectal lesion larger than 2 cm in diameter preoperatively assessed as a superficial neoplasm. By defining strict inclusion criteria, we excluded all TEM series that included preoperatively assessed malignant lesions because they were most probably biased by an extension of the inclusion criteria. The size limit requiring that lesions be larger than 2 cm was set according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines [34], which aims to achieve en bloc resection with no fragmentation.
With these restrictions in selection, heterogeneity of the results was kept within a reasonable frame, although some of the study samples included in this analysis were relatively small. We also performed additional analyses to adjust for these potential confounders, which indicated that their impact was null. By restricting the analysis to rectal lesions, we sought to limit any biases related to anatomic situations, which can influence the handling of lesions due to endoscope maneuverability restricted proximally to the rectum. As a consequence, the sensitivity analyses showed that no study had an influential effect on relative risk in the whole time frame.
A previous study comparing ESD with transanal excision (TAE) showed an advantage of ESD with respect to higher achievement of R0 en bloc resections [35]. Nonetheless, it is known that TEM is superior to TAE for the same reason, resulting in a significantly higher recurrence-free survival [36].
The TEM procedure remains the gold standard surgical treatment for rectal local excision. The pooled results of the current systematic review indicate that ESD for nonpedunculated superficial lesions of the rectum larger than 2 cm in diameter appears to be less effective than TEM, with an en bloc resection achieved for 88 % of patients compared with 99 % for TEM. Even more significantly, an R0 resection was achieved for 74 % of patients using ESD compared with 89 % using TEM. This difference was statistically significant. The apparently lower risk of recurrence shown in the ESD group was in fact not statistically significant, and in any case probably was due to the shorter follow-up period reported for the ESD series.
The ESD procedure is technically demanding with the currently available equipment and requires a significantly longer time to be completed. Yet the perioperative complication rate compared favorably with that of the TEM series, and the rate of abdominal surgery controlling complications was negligible.
Postoperative histology assessment demonstrated a much higher incidence of adenocarcinoma in the ESD series, which was attributable to a different way of classifying intramucosal lesions [37]. The rates of unpredicted invasive cancers treated in the two groups were comparable, but this required further surgery for oncologic reasons about four times more often in the ESD group due to the higher incidence of R1 resections than in the TEM group. In fact, a positive vertical margin after endoscopic resection is considered to be an indication for intestinal resection with lymph node dissection [34].
The high rate of further surgery for oncologic reasons after ESD also may explain the reduced risk of recurrence in this group. Although this could not be assessed through the analysis of the selected papers, the reduced incidence of abdominal surgery after TEM might be due to the fact that patients with a cancer extended to the submucosal layer who received an R0 full-thickness resection often refused to undergo intestinal resection with lymph node dissection due to the limited risk of metastasis.
An indisputable advantage of ESD for rectal lesions is that it does not entail the need for general anesthesia or a prolonged hospital stay, as usually is the case after full-thickness TEM resection, although this more often is a trend or based on a difference in the practice of surgeons and endoscopists. On the other hand, TEM supporters could argue that preoperative assessment of benign or noninvasive lesions still is suboptimal, so that even in this analysis, a consistent number of cases actually resulted in malignancy.
The intraoperative finding of deep wall invasion misdiagnosed preoperatively can significantly influence oncologic outcome. Moreover, the risk for infiltration of the vertical margin is the only risk factor for recurrence and the reason why EMR should be avoided in such circumstances [34]. Of extreme interest would have been the influence on anal continence and rectal function, sexual and urinary dysfunction, and quality of life, but the lack of sufficient data on these issues precluded further analyses.
Based on the evidence of the current review and analysis, we can conclude that TEM achieves a higher rate of en bloc and R0 excision. As a consequence, full-thickness rectal wall excision by TEM significantly reduces the need for further abdominal treatment. How these results will ultimately translate into common daily clinical practice remains unclear. No randomized head-to-head comparisons between TEM and ESD have been performed to date. Our review clearly highlights the need for a large randomized study to obtain unbiased results on the effectiveness and safety of these two strategies for patients with large rectal lesions preoperatively assessed as adenomas or noninvasive neoplasms.
References
Hermanek P, Gall FP (1986) Early (microinvasive) colorectal carcinoma: pathology, diagnosis, surgical treatment. Int J Colorectal Dis 1:79–84
Allaix ME, Arezzo A, Cassoni P et al (2012) Recurrence after transanal endoscopic microsurgery for large rectal adenomas. Surg Endosc 26:2594–2600
Ohkuwa M, Hosokawa K, Boku N et al (2001) New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy 33:221–226
Tamegai Y, Saito Y, Masaki N et al (2007) Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy 39:418–422
Higgins JPT, Green S (2010) Cochrane handbook for systematic reviews of interventions version 5.0.2 [updated September 2009]. The cochrane collaboration 2009. John Wiley & Sons, Ltd, Chichester
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Buess G, Kipfmüller K, Hack D et al (1988) Technique of transanal endoscopic microsurgery. Surg Endosc 2:71–75
Kudo S, Rubio CA, Teixeira CR et al (2001) Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy 33:367–373
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13–22
http://www.R-project.org. Accessed 1 Nov 2012
Fujishiro M, Yahagi N, Nakamura M et al (2006) Endoscopic submucosal dissection for rectal epithelial neoplasia. Endoscopy 38:493–497
Onozato Y, Kakizaki S, Ishihara H et al (2007) Endoscopic submucosal dissection for rectal tumors. Endoscopy 39:423–427
Ohya T, Ohata K, Sumiyama K et al (2009) Balloon overtube-guided colorectal endoscopic submucosal dissection. World J Gastroenterol 15:6086–6090
Iizuka H, Okamura S, Onozato Y et al (2009) Endoscopic submucosal dissection for colorectal tumors. Gastroenterol Clin Biol 33:1004–1011
Uraoka T, Ishikawa S, Kato J et al (2010) Advantages of using thin endoscope-assisted endoscopic submucosal dissection technique for large colorectal tumors. Dig Endosc 22:186–191
Ishii N, Itoh T, Horiki N et al (2010) Endoscopic submucosal dissection with a combination of small-caliber-tip transparent hood and flex knife for large superficial colorectal neoplasias including ileocecal lesions. Surg Endosc 24:1941–1947
Takeuchi Y, Uedo N, Ishihara R et al (2010) Efficacy of an endo-knife with a water-jet function (Flushknife) for endoscopic submucosal dissection of superficial colorectal neoplasms. Am J Gastroenterol 105:314–322
Yoshida N, Naito Y, Kugai M et al (2010) Efficient hemostatic method for endoscopic submucosal dissection of colorectal tumors. World J Gastroenterol 16:4180–4186
Saito Y, Uraoka T, Yamaguchi Y et al (2010) A prospective, multicenter study of 1,111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 72:1217–1225
Fusaroli P, Grillo A, Zanarini S et al (2009) Usefulness of a second endoscopic arm to improve therapeutic endoscopy in the lower gastrointestinal tract: preliminary experience: a case series. Endoscopy 41:997–1000
Niimi K, Fujishiro M, Kodashima S et al (2010) Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 42:723–729
Said S, Stippel D (1996) 10 years experiences with transanal endoscopic microsurgery. Histopathologic and clinical analysis. Chirurg 67:139–144
Cocilovo C, Smith LE, Stahl T et al (2003) Transanal endoscopic excision of rectal adenomas. Surg Endosc 17:1461–1463
Langer C, Liersch T, Suss M et al (2003) Surgical cure for early rectal carcinoma and large adenoma: transanal endoscopic microsurgery (using ultrasound or electrosurgery) compared to conventional local and radical resection. Int J Colorectal Dis 18:222–229
Neary P, Makin GB, White TJ et al (2003) Transanal endoscopic microsurgery: a viable operative alternative in selected patients with rectal lesions. Ann Surg Oncol 10:1106–1111
Schafer H, Baldus SE, Holscher AH (2006) Giant adenomas of the rectum: complete resection by transanal endoscopic microsurgery (TEM). Int J Colorectal Dis 21:533–537
Ganai S, Kanumuri P, Rao RS et al (2006) Local recurrence after transanal endoscopic microsurgery for rectal polyps and early cancers. Ann Surg Oncol 13:547–556
Doornebosch PG, Gosselink MP, Neijenhuis PA et al (2008) Impact of transanal endoscopic microsurgery on functional outcome and quality of life. Int J Colorectal Dis 23:709–713
Guerrieri M, Baldarelli M, de Sanctis A et al (2010) Treatment of rectal adenomas by transanal endoscopic microsurgery: 15 years’ experience. Surg Endosc 24:445–449
de Graaf EJ, Burger JW, van Ijsseldijk AL et al (2011) Transanal endoscopic microsurgery is superior to transanal excision of rectal adenomas. Colorectal Dis 13:762–767
Morino M, Allaix ME, Caldart M et al (2011) Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc 25:3683–3690
Allaix ME, Arezzo A, Caldart M et al (2009) Transanal endoscopic microsurgery for rectal neoplasms: experience of 300 consecutive cases. Dis Colon Rectum 52:1831–1836
Barendse RM, van den Broek FJ, Dekker E et al (2011) Systematic review of endoscopic mucosal resection versus transanal endoscopic microsurgery for large rectal adenomas. Endoscopy 43:941–949
Watanabe T, Itabashi M, Shimada Y et al (2012) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 17:1–29
Kiriyama S, Saito Y, Matsuda T et al (2011) Comparing endoscopic submucosal dissection with transanal resection for noninvasive rectal tumor: a retrospective study. J Gastroenterol Hepatol 26:1028–1033
Moore JS, Cataldo PA, Osler T, Hyman NH (2008) Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum 51:1026–1030
Schlemper RJ, Itabashi M, Kato Y et al (1998) Differences in the diagnostic criteria used by Japanese and Western pathologists to diagnose colorectal carcinoma. Cancer 82:60–69
Disclosures
Alberto Arezzo, Roberto Passera, Yutaka Saito, Taku Sakamoto, Nozomu Kobayashi, Naoto Sakamoto, Naohisa Yoshida, Yuji Naito, Mitsuhiro Fujishiro, Keiko Niimi, Tomohiko Ohya, Ken Ohata, Shinichi Okamura, Shinei Iizuka, Yoji Takeuchi, Noriya Uedo, Pietro Fusaroli, Marco Augusto Bonino, Mauro Verra, and Mario Morino have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arezzo, A., Passera, R., Saito, Y. et al. Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc 28, 427–438 (2014). https://doi.org/10.1007/s00464-013-3238-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3238-3