Abstract
Background and study aims
Classic endoscopic resection techniques (EMR and ESD) are limited to mucosal lesions. In the case of deeper growth into the gut wall and anatomic sites prone to perforation, the novel full-thickness resection device (FTRD®) opens a new dimension of possibilities for endoscopic resection.
Patients and methods
Sixty patients underwent endoscopic full-thickness resection (eFTR) at our institution. Safety, learning curve, R0 resection rate, and clinical outcome were studied.
Results
In 97% (58/60) of the interventions, the FTRD®-mounted endoscope reached the previously marked lesion and eFTR was performed (technical success). Full-thickness resection was achieved in 88% of the cases, with an R0 resection on histological examination in 79%. The clinical success rate based on follow-up histology was even higher (88%). Adverse events occurred in 7%. Appendicitis of the residual cecal appendix after eFTR of an adenoma arising in the appendix led to the only post-eFTR surgery (1/58, 2%). Minor bleeding at the eFTR site (2/58, 3%) and an eFTR performed accidently without proper prior deployment of the OTSC® (1/58, 2%) were successfully treated endoscopically. There was no secondary perforation or eFTR-associated mortality.
Conclusions
After specific training, eFTR is a feasible, safe, and promising all-in-one endoscopic resection technique. Our data show that eFTR allows complete resection of lesions affecting layers of the gut wall beneath the mucosa with a low risk of adverse events. However, our preliminary results need to be confirmed in larger, controlled studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are well-established and effective techniques for endoscopic resection of mucosal neoplasms along the entire gastrointestinal (GI) tract [1]. However, these procedures are limited to superficial lesions. Recently, an increasing number of studies have reported good results using submucosal tunneling endoscopic resection (STER) for small subepithelial tumors [2,3,4]. To date, STER is still limited to lesions in the esophagus, the cardia, and the proximal stomach. There are several conditions (e.g., non-lifting sign of a polyp due to scarring and fibrosis, difficult anatomic site) under which EMR and ESD are unsuitable resection techniques bearing a high risk of perforation [5,6,7]. Even though hybrid techniques such as full-thickness resection using a natural orifice transluminal endoscopic surgery (NOTES) access combined with laparoscopy known as laparoscopy-assisted endoscopic full-thickness resection (LAEFR) are feasible [8, 9]. These procedures are time consuming, not well standardized, and require like STER general anesthesia. Thus, such hybrid techniques were not implemented extensively among many centers. The armamentarium for sole endoscopic resection of submucosal lesions was limited until the introduction of the over-the-scope clip (OTSC®) in 2007 [10]. The OTSC® is now a well-established tool for the closure of gastrointestinal perforations and leaks. The efficacy of the OTSC® for various indications has already been proven in several studies [11,12,13,14]. Two case series on full-thickness snare resection followed immediately by OTSC® closure have shown excellent success rates [15, 16]. Based on the OTSC® as a powerful closure tool, a full-thickness resection device (FTRD®) has been developed. The FTRD® is an all-in-one device combining OTSC® closure of the GI wall with subsequent full-thickness resection. It allows full-thickness resection of GI tract lesions smaller than approximately 3 cm. Endoscopic full-thickness resection (eFTR) therefore has great potential in the treatment of lesions that previously could not be managed endoscopically and required a surgical approach. In principle, eFTR is indicated when resection of the submucosa or even the muscle layer is desirable at sites prone to perforation. There is only limited data on the performance of the FTRD® in humans so far [17,18,19]. As we described previously, the FTRD® system may be used for indications including previously untreated adenomas with a non-lifting sign, recurrent adenomas with scarring, and lesions at difficult locations with an increased risk of perforation (e.g., in a diverticulum or the appendix) [17]. Furthermore, the FTRD® may also be used to increase the diagnostic yield in early (T1) carcinoma, in situations with a contraindication to surgical resection, and for follow-up resection at the base of a malignant polyp priorly removed endoscopically. Other applications include resection of subepithelial tumors and diagnostic full-thickness resection in Hirschsprung’s disease. In addition to several case reports, Schmidt et al. [19] published the largest cohort of 25 patients in collaboration with our group [19]. We now present an analysis of 60 cases undergoing eFTR at our institution from data collected retrospectively and prospectively.
Materials and methods
Study design and patient characteristics
Over a 4-year period (June 2012–October 2016), 60 eFTRs were performed at our institution. The charts of the earlier patients were analyzed retrospectively. Starting in January 2015, the patients were prospectively included in the study. The following parameters were recorded: (1) indication for eFTR; (2) anatomical site of the lesion; (3) technical success (lesion reached with the endoscope and resected); (4) R0 resection rate (eFTR histology); (5) clinical success (based on follow-up histology); (6) adverse events; and (7) fate of the OTSC ®. The results of the first eight patients were previously included in Schmidt et al.’s cohort [19]. Another case was previously published by our group as a video case [17].
All patients gave their written informed consent for endoscopy, as well as for the publication of their medical data. The study was conducted in accordance with the declaration of Helsinki and was approved by the local ethics committee (BASEC 00284).
Indications for eFTR
Group 1 consisted of difficult recurrent adenomas with a non-lifting sign, whereas group 2 included patients who had polyps with a primary non-lifting sign. Evaluation by an experienced endoscopist considered traditional resection techniques for recurrent adenoma (EMR/ESD) to be high risk regarding perforation: eFTR was therefore performed. Group 3 consisted of patients undergoing eFTR in addition to piecemeal resection of large polyps. Piecemeal polypectomy was combined with eFTR if the lesion showed perforation-prone areas (non-lifting sign of the central part). Piecemeal EMR was then used initially to downsize the polyp in order to render it eligible for eFTR. Group 4 included patients with a T1 tumor who were not eligible for classic surgical resection. Group 5 consisted of patients with a submucosal lesion. eFTR was performed to increase the diagnostic yield if previous histology was uncertain or as a therapeutic option for complete removal of the lesion in patients not eligible for surgery. Group 6 included patients with an adenoma involving the appendix. eFTR was performed if classic EMR was considered high risk with respect to perforation or when there was a non-lifting sign. Group 7 included patients for follow-up resection of a malignant polyp after prior polypectomy. Group 8 consisted of patients undergoing eFTR over loop. Endoloops were used before the resection of large, pedunculated polyps, to prevent major bleeding. Due to the incomplete polypectomy above the endoloop and in order to prevent perforation, eFTR was used in the same session to complete the resection of the adenoma. The endoloop was usually pulled into the working channel of the endoscope to facilitate placing the lesion into the FTRD®. Group 9 consisted of cases with an adenoma involving a diverticulum. Classic polypectomy was rated as perforation-prone, so eFTR was carried out.
FTRD® and resection method
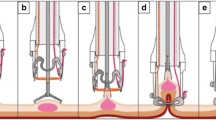
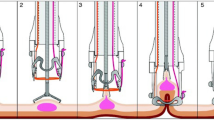
EFTR was performed as described previously [20]. The procedures were carried out by three experienced endoscopists. First, the target lesion is marked with electrocautery and/or ink injection and the endoscope is then retracted. The full-thickness resection device (FTRD, Ovesco® Endoscopy AG, Tübingen), consisting of an OTSC® mounted onto a transparent cap (Supplemental Fig. 7A) that also contains a hyperthermic snare, is fitted onto a therapeutic endoscope (Supplemental Fig. 7B). The endoscope with the FTRD® is advanced to the previously marked lesion (Supplemental Fig. 8, step 1). Grasping forceps are used to take hold of the target lesion (Supplemental Fig. 8, step 2) and carefully pull it into the plastic cap of the FTRD® (Supplemental Fig. 8, step 3). Immediately after deployment of the OTSC® (Supplemental Fig. 8, step 4), eFTR is performed using the hyperthermic snare within the plastic cap (Supplemental Fig. 8, step 5). The full-thickness specimen is retrieved and processed for histopathological examination. The endoscope is advanced again to check the immediate success of the intervention. For lesions in the proximal colon, guidewires and radiographic imaging are used to facilitate the advance of the FTRD®-mounted endoscope.
In our study, technical success was defined as reaching the lesion, deploying the clip, and performing eFTR. Clinical success was defined as histologically proven clear resection margins (R0 resection) after eFTR.
Procedures and postprocedural management
All endoscopies were performed under moderate to deep sedation with a non-anesthetist application of propofol (NAAP) and using standard Olympus® equipment (Olympus Medical Systems, Tokyo, Japan). Procedures were carried out using carbon dioxide insufflation by three experienced endoscopists who were certified in eFTR (endoscopists are required to complete a thorough training on animal models before using eFTR in a clinical setting). Procedural single-shot intravenous antibiotics (broad-spectrum cover for gram-negative and gram-positive bacteria) were administered in 55 of the 58 cases. The resection was performed either during a short hospital stay or on an outpatient basis, depending on the difficulty of the intervention itself, the patient’s age, and comorbidities. Patients resumed a normal diet on the day after the procedure.
Follow-up
All patients came for clinical follow-up. The histology of the resected lesion determined the time to follow-up endoscopy, in accordance with current national and international guidelines [21]. In cases of R0 resection without evidence of carcinoma, the time to follow-up endoscopy was 3–5 years. In cases of incomplete resection (R1), we removed the clips after 4–6 weeks using a clip cutter device (remOVE® OVESCO, Tübingen) and completed the resection. In cases of T1 carcinoma requiring further treatment, follow-up surgery was scheduled promptly.
Results
Patient characteristics, indications, and anatomic sites for eFTR
Over a 4-year period (June 2012–October 2016), 60 patients underwent therapeutic eFTR at our institution. The first eight cases were published previously in collaboration with Schmidt et al. [19]. Patients were allocated to one of the indication groups given in the methods section and summarized in Table 1. Twenty-two patients (37%) had polyps that were recurrent adenomas with a non-lifting sign after previous incomplete polypectomy, while two patients (3%) had adenomas with a primary non-lifting sign on saline injection (Figs. 1A–D, 2A–D, supplemental Fig. 6A–C). In ten cases (17%), eFTR was performed at the non-lifting base after extensive piecemeal resection of a spreading adenoma. Two polyps (3%) in a diverticulum were resected using the FTRD® [17]. Four polyps (6.7%) arising from the cecal appendix were treated with eFTR (Fig. 3A–F). Five (8.3%) patients had submucosal lesions and underwent eFTR (Fig. 4A–D); histopathological examination of the full-thickness specimen of these submucosal lesions revealed three neuroendocrine tumors (NETs), one gastrointestinal stromal tumor (GIST), and one inflammatory fibroid polyp. Seven patients underwent eFTR for early carcinoma (12%) (Fig. 5A–C) and six patients (10%) had follow-up resection of a malignant polyp. In two cases (3.3%), we conducted eFTR over endoloop.
A Adenoma within cecal appendix; B adenoma is pulled into the FTRD®; C resection site after endoscopic full-thickness resection (eFTR) with OTSC® in place; D full-thickness resection specimen: visible adenoma within cecal appendix; E resection specimen: visible adenoma within cecal appendix; F eFTR of adenoma within residual cecal appendix after a surgical appendectomy years ago. Visible surgical staples in the endoscopic resection specimen
The anatomic resection sites were distributed as follows: esophagogastric junction (2, 3.3%), stomach (3, 5%), cecum (5, 8.3%), cecal appendix (4, 6.7%), ascending colon (15, 25%), transverse colon (1, 1.7%), descending colon or sigmoid (16, 26.7%), and rectum (14, 23.3%), see Table 1.
Procedures and success rates
We were able to reach the marked target lesion with the FTRD®-mounted endoscope and perform eFTR (technical success) in 97% of the cases. It was not possible to reach the target lesion in two cases (3%). In one patient, the endoscope could not be advanced far enough into the ascending colon because of a fixed sigmoid. In the other case, even dilation to 20 mm did not permit the scope to pass a diverticular stricture of the sigmoid. On endoscopic evaluation, full-thickness and en bloc resection was achieved in 91.4% (53 cases), while it was not clear whether the resection had been truly full-thickness in 5%. Based on histopathological examination of the eFTR specimen, full-thickness resection was achieved in 88% (51/58). Complete (R0) resection was accomplished in 79% (46/58). The main reasons for incomplete (R1) resections were eFTR of recurrent polyps with non-lifting sign (n = 9), T1 tumors (n = 2), and submucosal tumors (n = 1). In case of incomplete resection, the OTSC® was removed and residual adenoma within the pseudopolyp was resected using either an EMR or eFTR technique. The two patients with incomplete resection and malignant tumor had subsequent surgical resections. Interestingly, five of the cases considered R1 on eFTR histology were restaged to primary R0 resection on the basis of follow-up histology (obtained either by follow-up endoscopic resection after clip removal or by surgical resection). The re-allocation based on follow-up histology improved the clinical success rate to 88% (51/58).
The mean diameter of the resected specimens was 24 mm (range 10–35 mm). The median procedure time was 60 min (range 15–177 min), see Table 2.
Adverse events and technical issues
There were four (7%) adverse events (two major, two minor) related to the eFTR in our 58 patients. One patient (2%) developed appendicitis of the residual cecal appendix after eFTR of an adenoma within the proximal appendix. Laparoscopic appendectomy of the residual appendix was performed. Two weeks after eFTR, minor bleeding (hemoglobin drop <2 g/l) occurred at the resection site. Sustained hemostasis was achieved endoscopically after the injection of saline-diluted epinephrine and the application of a hemoclip. Another patient suffered from self-limiting hematochezia after eFTR of a large rectal polyp (minor bleeding). In one patient (2%), eFTR was performed accidently without proper prior deployment of the OTSC®. The 2.5-cm full-thickness defect was then closed using three OTSC®s (one 14/6 t, two 11/6 t). Sufficient closure was achieved as demonstrated by contrast-enhanced computed tomography and the patient recovered fully. No secondary perforation occurred after eFTR, as shown in Table 2.
Malfunction of the resection snare occurred in 3(5%) cases. For all three snare malfunctions, secondary complete resection with a standard polypectomy snare during the same endoscopy was achieved (Table 2). The adverse events occurred evenly distributed among the chronologically listed cases suggesting no existence of a learning curve for performing the procedure.
Follow-up and fate of the OTSC®
All patients attended clinical follow-up. The mean follow-up time was 16 months (range 2–54 months). No adverse events occurred during the follow-up period. There was no recurrence of adenomatous tissue at the site of eFTR after prior complete resection (R0) for those patients with an available endoscopic follow-up (34/58, 59%). Placement of the FTRD® clip usually resulted in a narrowing of the gut lumen, but there was no clinically relevant stenosis. The fate of 24 (41%) clips is unknown, as these patients have not yet had follow-up endoscopy or imaging. Thirty-four patients (59%) have undergone follow-up endoscopy to date. Four (12%) of these 34 OTSC®s were still in place, 26 (77%) of the clips fell off spontaneously, three (9%) were removed endoscopically, and one (3%) was removed during secondary surgical resection, see Table 2.
Discussion
After the clinical introduction of endoscopic full-thickness resection (eFTR), we present data from 60 patients in the largest single-center cohort so far. Our data underline that eFTR has become a valuable tool for various conditions where classic endoscopic resection techniques such as EMR and ESD have failed or were considered to be high risk with respect to perforation. Compared to the presently available possibilities to achieve endoscopic full-thickness resection including NOTES and LAEFR-based techniques, eFTR offers a much less sophisticated and more timesaving procedure, which can be performed on an outpatient basis in NAAP.
The FTRD® system is designed in such a way that it allows complete and reliable closure of the GI wall by applying an OTSC® immediately before snare resection of the enclosed gut wall. In comparison with simple OTSC® closure after full-thickness resection [15, 22, 23], no free perforation of the gut occurred at any time during the eFTR procedure.
There are several specific conditions, such as adenomas at difficult anatomic sites, in which eFTR might be a valuable alternative to prevent complication-prone and expensive surgery. The risk of colonic perforation in case of intra-diverticulum lesions [24, 25] is elevated during standard polypectomy procedures due to the lack of a muscular coat beneath the diverticulum. The same is true for polypectomy within the cecal appendix; in the past, therefore, these lesions have been managed by primary laparoscopy [26, 27]. We have previously shown that the FTRD® is a useful tool to avoid surgery under such conditions [17].
Indications
The most frequent eFTR indications in our cohort were adenomas with a primary non-lifting sign and recurrent adenomas displaying a non-lifting sign after prior polypectomy. The perforation rate of EMR and ESD in the case of failed prior submucosal fluid injection increases up to nearly 15% [5, 6]. As the histology of such lesions is frequently not conclusive prior to histopathological evaluation of the entire specimen (high grade dysplasia (HGD), T1 carcinoma), perforation additionally carries the risk of tumor cell seeding into the peritoneal cavity. eFTR may offer an alternative endoscopic treatment option for difficult, perforation-prone lesions, as we did not observe any perforations or leaks in our cohort after proper OTSC® deployment. The only perforation occurred in one of the first eFTR cases, when a technical problem led to an incomplete OTSC® release prior to eFTR. When the OTSC® was deployed correctly, we observed no perforations on eFTR. However, prospective controlled trials are needed to confirm these findings and to decide whether EMR/ESD or eFTR is superior for such perforation-prone lesions. This is most important for lesions arising from the appendicular orifice given the fact that one out of four patients developed post-interventional appendicitis in our cohort. Yet, eFTR is less invasive than primary surgical ileocecal resection requiring a sutured anastomosis and may therefore be a valuable alternative to primary surgical resection in selected patients with multiple comorbidities. In case of a post-eFTR appendicitis, a less invasive laparoscopic appendectomy may resolve the issue. However, this issue should be addressed in a controlled trial versus standard surgery.
From our own experience and the experience of other centers, we assume that only approximately 10% of the patients undergoing eFTR of the appendicular orifice will experience post-interventional appendicitis. Even in the relatively rare event of post-eFTR appendicitis, a less invasive laparoscopic appendectomy can permanently resolve the issue.
Since the FTRD® offers a clip-before-resection technique, the theoretical risk of intra-abdominal tumor cell seeding upon resection of malignant lesions is substantially reduced. Alongside the treatment of adenomas displaying a non-lifting sign in risky anatomic sites, eFTR might be useful for further diagnostic reasons (depth of submucosal infiltration and confirmation of complete resection, R0) with T1 tumors, as well as being a therapeutic tool in low-risk circumstances (well-differentiated tumor and submucosal infiltration <1000 μm) and inoperable patients. In the future, the FTRD® may also play a role in eFTR of selected subepithelial lesions (e.g., NETs) of limited size and for harvesting full-thickness specimens in the diagnostic investigation of neurogenic GI diseases involving ganglia cells, such as Hirschsprung’s disease.
Procedures
Our median procedure duration was 60 min, with a fairly wide range. The longer procedure times were mostly due to the time-consuming advancement of the FTRD®-fitted endoscope to target lesions in more proximal anatomic sites and not to the procedure itself. In addition to the relatively large FTRD® (outer diameter 21 mm) restricting visibility, the plastic sleeve surrounding the endoscope and the polypectomy snare makes advancing the endoscope considerably more difficult. Accessing the target lesion may be particularly challenging in the case of extensive diverticulosis and gut strictures, proximal lesions, and fixed colonic sections. Cecal intubation may be impossible with the FTRD® in patients with extensive diverticulosis, as we found in the two cases where we were unable to reach the target lesion. Nevertheless, the technical success rate in our cohort was 97%. In the case of a proximal colonic lesion, after marking the lesion we placed a guidewire in the cecum to facilitate re-endoscopy with the mounted FTRD®. Technical success was always achieved if the mounted FTRD® reached the target lesion.
Even though the primary use of the FTRD® is in the lower GI tract, we successfully performed five eFTRs in the upper GI tract. Due to the size of the FTRD® cap, passage through the esophagus may cause local injury and is only possible in a very select patient population with an easy-to-pass upper esophageal sphincter. The manufacturer has recently introduced a plastic cap with the exact dimensions of the FTRD® to allow an endoscopic feasibility trial.
Completeness of resection
Within our cohort, the rate of histologically clear resection margins (R0 resection rate) was reasonably high at 79% (46/58). This is in agreement with the previous dataset published by Schmidt et al. [19]. However, in five cases with primary R1 eFTR, follow-up histology revealed no residual elements of the lesion, suggesting primary R0 resection. The clinical success rate therefore improved to 88% (51/58) based on the follow-up histology of the eFTR site (follow-up resection carried out either endoscopically or surgically). We suggest that this discrepancy is due to electrocautery damage of the eFTR specimen making it difficult to assess whether the margins are clear. We therefore consider that the clinical success rate might be underestimated when based on the primary histology (R0 rate) after eFTR.
Interestingly, the rate of R0 resection was not as low as expected in the adenoma piecemeal resection group. In this particular group, clear resection margins were confirmed on all histopathological specimens (9/9). On the other hand, R1 resections occurred predominantly in the non-lifting/recurrent adenoma group. In this group, 17% (4/24) were not R0. The other two cases with R1 resection were a submucosal tumor (one) and a T1 carcinoma (one).
Resection specimen
The main factor impeding full-thickness resection is an inflexible GI wall preventing the retrieval of all wall layers into the plastic cap of the FTRD®. This may be the case if fibrosis and scarring are present in the lesion itself or in the surrounding tissue. Due to its fixed retroperitoneal anatomy, the rectum is also a site that presents difficulties in achieving a full-thickness resection. In the case of a failed full-thickness resection, the OTSC® may be removed endoscopically and a second eFTR of the residual lesion may be attempted.
The median diameter of full-thickness specimens in our cohort was 24 mm, ranging from 10 to 35 mm. This median specimen diameter agrees with previous work by Schmidt et al. Since the final diameter is determined by the amount of tissue retracted into the plastic cap of the FTRD®, the final sample size greatly depends on the tissue properties of the target lesion and its surroundings. Compared with healthy tissue (e.g., diagnostic eFTR), fibrotic tissue and scarring will not allow a large sample size. Fibrosis and chronic inflammation will not only reduce the sample diameter but, in some cases, will also prevent a full-thickness specimen. We were therefore not able to achieve full-thickness resection in 12% (7) of the cases. However, since six of these seven cases were patients with non-lifting adenomas or recurrent adenomas after prior polypectomy, a full-thickness resection including the deep muscle layers and the serosa was not necessary.
Adverse events and technical issues
The overall adverse event rate in our patient population was 7% (4/58). The major adverse event was a full-thickness resection following incomplete OTSC® release. The ensuing perforation was then closed by applying three OTSC®s. A second patient suffered from severe inflammation of the appendix remaining after eFTR of a polyp arising from the appendicular orifice. The patient recovered fully after laparoscopic appendectomy and endoscopic treatment of minor bleeding from the resection site. These data suggest that eFTR is a valuable option for well-chosen indications. Nevertheless, it is an invasive method: the endoscopist needs to be aware of this fact and ready to treat any adverse events that may occur. The following adverse advents are theoretically possible: (i) gut injury, ranging from mucosal laceration to perforation, and bleeding caused by the plastic cap; (ii) tissue damage, burns, bleeding, and even colonic gas explosion caused by the resection snare; (iii) insufficiency of the OTSC® closure; (iv) obstruction of the gut lumen; (v) injury or resection of surrounding tissue or organs. It is likely that these adverse events will occur if sufficiently large numbers of procedures are performed. The even distribution among the chronologically listed cases suggests no existence of a learning curve for performing the procedure after proper training.
Fifty-five patients received at least a single-shot broad-spectrum antibiotic during the procedure. Three patients did not receive any procedural antibiotics. Except for the patient who developed an acute inflammation of the appendix remnant, there were no infections after eFTR. This fact underlines that OTSC® closure is tight and a single shot of antibiotics can prevent any theoretical bacterial infection caused by the eFTR. Since the clip penetrates the gut wall completely in most cases, we recommend at least single-shot antibiotic prophylaxis, although further studies are needed to verify this recommendation.
Follow-up
After R0 polypectomy of adenomas, patients were scheduled according to international guidelines for post-polypectomy colonoscopy surveillance [21]. In the patients undergoing follow-up endoscopy (59%), three-quarters of the OTSC®s fell off spontaneously without causing any problems. Removing the OTSC® by means of a clip cutter device is endoscopically feasible, and was performed in cases of R1 resection in order to allow resection of remaining adenomatous tissue. However, clip removal is not necessary in the majority of cases. For all patients undergoing eFTR for polyp resection, no adenoma recurrence was observed in any patient with an endoscopic follow-up at the eFTR site following an initial R0 resection.
Conclusions
In conclusion, after specific training, endoscopic full-thickness resection is a feasible, safe, and promising resection technique. It allows complete resection of lesions affecting layers of the gut wall beneath the mucosa, without the risk of perforation. In the future, eFTR may become a valuable alternative to a surgical approach in cases where endoscopic resection was previously considered impossible. However, we feel that in the future, randomized controlled trials are needed to confirm our results, including the safety of the method. Introduction of this novel method should be accompanied by a prospective registry to acquire reliable information on the indication, outcome, and adverse events.
Abbreviations
- EMR:
-
Endoscopic mucosal resection
- ESD:
-
Endoscopic submucosal dissection
- STER:
-
Submucosal tunneling endoscopic resection
- LAEFR:
-
Laparoscopy-assisted endoscopic full-thickness resection
- NOTES:
-
Natural orifice transluminal endoscopic surgery
- GI:
-
Gastrointestinal
- OTSC® :
-
Over-the-scope clip
- FTRD® :
-
Full-thickness resection device
- eFTR:
-
Endoscopic full-thickness resection
References
Maguire LH, Shellito PC (2014) Endoscopic piecemeal resection of large colorectal polyps with long-term followup. Surg Endosc 28:2641–2648
Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY (2014) Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 28:524–530
Lu J, Jiao T, Li Y et al (2015) Heading toward the right direction–solution package for endoscopic submucosal tunneling resection in the stomach. PLoS ONE 10:e0119870
Eleftheriadis N, Inoue H, Ikeda H, Onimaru M, Maselli R, Santi G (2016) Submucosal tunnel endoscopy: peroral endoscopic myotomy and peroral endoscopic tumor resection. World J Gastrointest Endosc 8:86–103
Kuroki Y, Hoteya S, Mitani T et al (2010) Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol 25:1747–1753
Sakamoto T, Saito Y, Matsuda T, Fukunaga S, Nakajima T, Fujii T (2011) Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc 25:255–260
Moss A, Williams SJ, Hourigan LF et al (2015) Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut 64:57–65
Abe N, Takeuchi H, Yanagida O et al (2009) Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc 23:1908–1913
Cho WY, Kim YJ, Cho JY et al (2011) Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection–14 human cases. Endoscopy 43:134–139
Kirschniak A, Kratt T, Stuker D, Braun A, Schurr MO, Konigsrainer A (2007) A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest endosc 66(1):162–167
Honegger C, Valli P, Wiegand N, Bauerfeind P, Gubler C (2016) Establishment of over-the-scope-clips (OTSC®) in daily endoscopic routine. United Eur Gastroenterol J 5(2):247–254
Weiland T, Fehlker M, Gottwald T, Schurr MO (2013) Performance of the OTSC system in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc 27:2258–2274
Voermans RP, Le Moine O, von Renteln D et al (2012) Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol 10:603–608
von Renteln D, Schmidt A, Vassiliou MC, Rudolph HU, Gieselmann M, Caca K (2009) Endoscopic closure of large colonic perforations using an over-the-scope clip: a randomized controlled porcine study. Endoscopy 41:481–486
Sarker S, Gutierrez JP, Council L, Brazelton JD, Kyanam Kabir Baig KR, Monkemuller K (2014) Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy 46(9):758–761
von Renteln D, Schmidt A, Vassiliou MC, Rudolph HU, Caca K (2010) Endoscopic full-thickness resection and defect closure in the colon. Gastrointest Endosc 71:1267–1273
Valli PV, Kaufmann M, Vrugt B, Bauerfeind P (2014) Endoscopic resection of a diverticulum-arisen colonic adenoma using a full-thickness resection device. Gastroenterology 147:969–971
Schmidt A, Meier B, Caca K (2015) Endoscopic full-thickness resection: current status. WJG 21:9273–9285
Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K (2015) Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy 47:719–725
Schurr MO, Baur F, Ho CN, Anhoeck G, Kratt T, Gottwald T (2011) Endoluminal full-thickness resection of GI lesions: a new device and technique. MITAT 20:189–192
Hassan C, Quintero E, Dumonceau JM et al (2013) Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 45:842–851
Fahndrich M, Sandmann M (2015) Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy 47:76–79
Monkemuller K, Peter S, Toshniwal J et al (2014) Multipurpose use of the ‘bear claw’ (over-the-scope-clip system) to treat endoluminal gastrointestinal disorders. Dig Endosc 26:350–357
Xu J, Yang L, Guo Y, Zhao D, Wang L, Bai L (2010) Perforation of sigmoid diverticulum following endoscopic polypectomy of an adenoma. BMJ Case Rep. doi:10.1136/bcr.07.2009.2077
Barr YR, Brazowski E, Leider-Trejo L (2006) Villous adenoma in a perforated colonic diverticulum. Int J Colorectal Dis 21:282–284
Adrales GL, Harold KL, Matthews BD, Sing RF, Kercher KW, Heniford BT (2002) Laparoscopic “radical appendectomy” is an effective alternative to endoscopic removal of cecal polyps. J Laparoendosc Adv Surg Tech A 12:449–452
Denoel C, Legrand MJ, Heymans O, Kunsch JM (2001) Isolated adenomatous polyposis of the appendix: report of a case. Dis Colon Rectum 44:1709–1710
Acknowledgements
We sincerely thank PD Dr. Christoph Gubler for his assistance in the performance of some interventions.
Author contributions
PVV: Editing of results and manuscript, performance of endoscopies. JM: Critical reading and editing of the manuscript. PB: Revision of the manuscript, performance of endoscopies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
P. V. Vall, J. Mertens, and P. Bauerfeind have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
464_2017_5676_MOESM1_ESM.tif
Supplementary material 1 (TIFF 1035 kb). Supplemental Figure 6A Recurrent adenoma after prior polypectomy; B Full-thickness resection specimen, peritoneal face; C Full-thickness resection specimen, adenoma face with all marks included within the resection limits.
464_2017_5676_MOESM2_ESM.tiff
Supplementary material 2 (TIFF 1321 kb). Supplemental Figure 7A Full-thickness resection device (FTRD®), including a hyperthermic snare, running along a standard endoscope; B OTSC® mounted onto a transparent cap containing the resection snare, then fitted on the tip of the endoscope.
464_2017_5676_MOESM3_ESM.tif
Supplementary material 3 (TIFF 534 kb). Supplemental Figure 8 (kindly provided by Ovesco® Endoscopy AG, Tübingen), step 1: After mounting the FTRD®, the endoscope is advanced to the previously marked lesion; step 2: The target lesion is grabbed with a grasping forceps; step 3: The target lesion is carefully pulled into the plastic cap of the FTRD®, step 4: The OTSC® is deployed; step 5: The eFTR is performed using the hyperthermic snare within the plastic cap.
Rights and permissions
About this article
Cite this article
Valli, P.V., Mertens, J. & Bauerfeind, P. Safe and successful resection of difficult GI lesions using a novel single-step full-thickness resection device (FTRD®). Surg Endosc 32, 289–299 (2018). https://doi.org/10.1007/s00464-017-5676-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5676-9