Abstract
Background
The advantages of reduced-port laparoscopic surgery (RPLS) for rectosigmoid cancer treatment have been disputed. This study evaluated the outcomes of RPLS compared to conventional laparoscopic surgery (CLS) for rectosigmoid cancer.
Methods
Data from 211 patients who underwent a selective sigmoidectomy or anterior resection from August 2011 to June 2014 at a single institution were collected and analyzed via propensity score matching. Operative outcomes, inflammatory responses, pain intensity, oncologic outcomes, quality of life, and cosmetic results were compared between groups.
Results
After matching, 96 patients (48 CLS and 48 RPLS) were evaluated. Sixteen RPLS cases underwent single-incision laparoscopic surgery (SILS), and 32 underwent single-incision plus one port laparoscopic surgery (SILS + 1). Baseline clinical characteristics were comparable between the RPLS and the CLS groups. Morbidity, pathologic outcomes, and 3-year disease-free survival and overall survival rates were also comparable between the 2 groups. Compared with the CLS group, the RPLS group had a shorter total incision length (p < 0.001); shorter time to liquid diet (p = 0.027), ambulation (p = 0.026), and discharge (p < 0.001); and lower visual analogue scale scores during mobilization at postoperative days 3–5 (p < 0.05). The total operation times, C-reactive protein levels at 24 h and 96 h, and interleukin-6 levels at 24 h postoperatively were significantly lower in the SILS + 1 group than those in the CLS and SILS groups (p < 0.05). Compared with the CLS group, the RPLS group showed better social functioning at 6 months postoperatively (p = 0.011). The SILS and SILS + 1 groups showed similar cosmetic results, and both groups showed better results than the CLS group (p < 0.001).
Conclusions
RPLS for rectosigmoid cancer is feasible, with short-term safety and long-term oncological safety comparable to that of CLS. Better cosmesis and accelerated recovery can be expected. SILS + 1 is a better choice than CLS or SILS for rectosigmoid cancer because it minimizes invasiveness and reduces technical difficulties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Conventional laparoscopic surgery (CLS) is a minimally invasive technique that has been shown to be as safe as open surgery for colorectal cancer [1, 2]. Reduced-port laparoscopic surgery (RPLS) is also a minimally invasive technique that reduces the length of the abdominal incision by reducing the number and/or size of the trocars used. RPLS includes both single-incision laparoscopic surgery (SILS) and single-incision plus one port laparoscopic surgery (SILS + 1) [3]. SILS is performed entirely through one extraction site, typically the umbilicus, which can conceal a surgical scar [4, 5]. SILS offers potential benefits over CLS, including a reduced risk of trocar-related complications, reduced postoperative pain, and improved convalescence and cosmetic results [4–6]. However, SILS is technically challenging because of limited instrument movement, loss of triangulation, and limited in-line viewing [6–9]. Particularly for rectosigmoid cancer, performing a rectal transection and double-stapling anastomosis intracorporeally through the umbilical incision is difficult [4, 9, 10]. To minimize the abdominal trauma and these technical difficulties in the treatment of rectosigmoid cancer, SILS + 1, which includes an additional port in the right-lower quadrant, has gained increasing attention from surgeons [10, 11]. However, only a few studies have reported on the differences in short-term outcomes among the SILS, SILS + 1, and CLS methods [12], and no study has reported on the differences in long-term oncologic outcomes, pain intensity, postoperative inflammatory responses, quality of life (QOL), or cosmetic results among the 3 methods.
This study aimed to evaluate the short-term safety, long-term oncologic safety, pain intensity, postoperative inflammatory responses, QOL, and cosmetic results of RPLS compared with CLS for rectosigmoid cancer. In addition, we aimed to determine the potential risks and benefits among the SILS, SILS + 1, and CLS methods by comparing SILS + 1 with CLS, and SILS + 1 with SILS.
Materials and methods
Patients
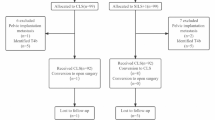
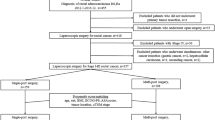
This study was based on the colorectal cancer database of prospectively collected data at the authors’ hospital. Overall, 295 patients had confirmed rectosigmoid cancer from August 2011 to June 2014, 211 of those met the inclusion criterion (Table 1), and 184 patients were analyzed (Fig. 1). The allocation depended on the patient’s willingness to participate. The patients in both groups were treated by the same surgeon, who had performed ≥100 successful laparoscopic colorectal resections and ≥10 successful RPLS colorectal resections. All patients were informed about the possible advantages and limitations of RPLS and signed an informed consent form. Data regarding demographics and clinical information were obtained from the prospective database. This study was approved by the Ethics Committee of Nanfang Hospital.
The patients in the RPLS group (n = 48) were compared with the patients in the CLS group (n = 136) to assess imbalances of covariates, and 1:1 propensity score matching was then performed to control for confounding factors and to obtain more reliable evidence. The matching covariates included age, gender, body mass index (BMI), American Society of Anesthesiologists score, surgical approaches (sigmoidectomy or anterior resection), tumor diameter, and pathologic tumor-node-metastasis stage according to the 7th edition of the AJCC Cancer Staging Manual [13]. These 2 groups were compared based on the following variables: perioperative outcomes; pathologic and oncologic outcomes; QOL (measured using the European Organization for Research and Treatment of Cancer [EORTC] QLQ-C30 [14] and the EORTC QLQ-CR29 [15] preoperatively and at 1 month, 3 months, and 6 months postoperatively); and cosmetic results (measured using the Body Image Questionnaire [16]) at 1 month, 3 months, and 6 months postoperatively and the Photo Series Questionnaire (Fig. 2) at 6 months postoperatively [16]. Briefly, the patients who completed the Photo Series Questionnaire were first asked to grade their own surgical scar on a scale from 1 to 10; then asked to grade photographs of other surgical scars after CLS (Fig. 3A), SILS + 1 (Fig. 3B), and SILS (Fig. 3C); and finally asked to grade their own scar again. Next, they were asked for their preference for one of the three surgical approaches if, hypothetically, they had the choice. In another question, they were asked for their preference in a hypothetical situation where the only differences among SILS, SILS + 1, and CLS were the level of difficulty in postoperative morbidity evaluation and management.
Surgical techniques
For the SILS group, a 4-cm transverse suprapubic incision was made 3 cm above the pubic symphysis. Two 12-mm trocars and two 5-mm trocars were inserted through the glove fingers. Then, the homemade multichannel device, comprising a soft tissue retractor with a surgical glove, was inserted (Fig. 4). For the SILS + 1 group, an initial 4-cm periumbilical transverse incision was made, and the homemade multichannel device was inserted. Two 12-mm trocars and one 5-mm trocar were inserted through the glove fingers. One 12-mm trocar was placed in the right-lower quadrant. For the CLS group, 5 ports were used, including a camera port. The size of the left-upper, left-lower, and right-upper ports was 5 mm. The size of the umbilicus and right-lower ports was 12 mm. The surgery technique used for the SILS and SILS + 1 groups was identical to the technique used in the CLS group. The rectosigmoid was mobilized using a medial-to-lateral approach. Mobilization of the splenic flexure was not performed routinely except in cases with a lack of redundancy in the sigmoid colon. Adding ports or converting to open surgery was allowed at the surgeon’s discretion because of technical difficulties. The distal rectum was dissected by inserting a linear stapling device through the suprapubic incision in the SILS group and through the right-lower channel in CLS and SILS + 1 groups. For the SILS + 1 and CLS groups, a drainage tube was placed in the pelvic cavity through the right-lower quadrant channel, but the drain was not routinely placed in the SILS group. Only standard straight laparoscopic instruments were used.
Perioperative management and follow-up
For the RPLS and CLS groups, perioperative management, laboratory blood tests, and discharge criteria were the same.
Perioperative management
Postoperatively, patient-controlled opioid-based intravenous analgesia (PCIA) was routinely administered immediately after surgery in the recovery room and discontinued on postoperative day (POD) 2. Additional analgesics were allowed in cases of breakthrough pain. The patients were encouraged to start a liquid diet after they had passed gas and a soft diet after they had defecated. The times to liquid diet and soft diet were at the discretion of the surgeon and influenced by the willingness of the patient. The drain was removed at the surgeon’s discretion based on the amount of drainage and the properties of the drained fluid. Patients were discharged when they were able to tolerate a soft diet and ambulate independently.
Inflammatory responses
White blood cell (WBC), C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) levels were used as parameters for the extent of inflamed and damaged tissue. Peripheral blood and serum (Vacutainer Systems, Chengdu, China) were collected preoperatively (baseline) and at 4, 24, and 96 h after surgery. All samples were collected within 15 min of the precise postoperative time. Transportation of the serum to the laboratory was accomplished within 15 min. Serum IL-6, CRP, and TNF-α samples were obtained by centrifugation for 10 min at 3000 rpm at 4 °C. The serum samples were subsequently stored at −20 °C until assay. WBC was analyzed directly with full-blood samples. The IL-6 concentrations in serum were measured using a commercially available electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany). CRP was measured by the immunoturbidimetric method (Roche Diagnostics GmbH, Mannheim, Germany). TNF-α was measured with a chemiluminescent immunoassay (SIEMENS, Gwynedd, UK). The WBC count was determined with an automated hematology analyzer (XE-2100, Sysmex, Kobe, Japan).
Follow-up
Follow-up visits were scheduled at 1 month and 3 months postoperatively and then every 3 months for the first 2 years and every 6 months for the next 3 years. A colonoscopy and abdominal and pelvic computed tomography scans were performed every 6 months for the first 2 years and then annually for the next 3 years.
Statistics
Categorical variables were described as numbers with percentages and were compared using the chi-square test (χ2) or Fisher’s exact test. Continuous variables were described as the mean ± standard deviation and were compared using Student’s t test or the Mann–Whitney U test. The disease-free survival (DFS) rate was calculated from the time of surgery to the time of either local or distant progression or to the time of the last known follow-up without evidence of recurrence. The overall survival (OS) was calculated from the time of surgery to the time of the last visit for a regular follow-up. The Kaplan–Meier method was used to estimate survival rates, and the log-rank test was used to analyze the OS and DFS differences between the two groups. A linear regression model and the Kruskal–Wallis H test were performed as needed. A p value of less than 0.05 was considered statistically significant. The statistical analysis was performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA).
For the EORTC QLQ-C30 and QLQ-CR29, scores were reported on a scale from 0 to 100 according to the EORTC Scoring Manual [15, 17]. On the function scales, a higher score indicated better function. On the symptom scales, a higher score indicated more severe symptoms.
Results
Baseline clinical characteristics of the patients
Baseline characteristics of the prematched groups are shown in Table 2. After propensity score matching, no significant differences were found between the RPLS and CLS groups or between the SILS + 1 and CLS groups (Table 2).
Perioperative outcomes
Perioperative outcomes are shown in Table 3. No intraoperative complications, mortality, or readmission within 30 days of surgery occurred in the RPLS and CLS groups. Four patients in the RPLS group had ports added during surgery (2 patients in the SILS group had 1 port added and 2 patients in the SILS + 1 group had 3 ports added) because of surgical difficulties, but the differences between the RPLS and CLS groups were not statistically different (P = 0.117). No procedure was converted to open surgery. No additional analgesics were used. There were no significant differences in morbidity within 30 days of surgery (p = 0.463) or in the Clavien-Dindo classification (p = 0.482) between the RPLS and CLS groups. Using a linear regression model, we found that the length of the minilaparotomy had a direct correlation with BMI (slope = 0.170, R 2 = 0.300, 95 % confidence interval = 0.117–0.223, p < 0.001) and tumor diameter (slope = 0.330, R 2 = 0.214, 95 % confidence interval = 0.200–0.459, p < 0.001). Time to liquid diet (p = 0.027) and ambulation (p = 0.026), length of hospital stay (p < 0.001), and length of total incision (p < 0.001) were significantly shorter in the RPLS group than in the CLS group. The total operation time in the SILS + 1 group was significantly shorter than that in the CLS (p = 0.023) and SILS (p = 0.018) groups. The length of total incision (p < 0.001) and length of hospital stay (p < 0.001) were significantly shorter in the SILS + 1 group than in the CLS group.
At rest, no significant difference was observed in the visual analogue scale (VAS) between the RPLS and CLS groups or among the SILS, SILS + 1, and CLS groups (Fig. 5A, C). During mobilization, the VAS was significantly higher in the CLS group than in the SILS and SILS + 1 groups on POD 3–5 (Fig. 5B, D).
Visual analogue scale (VAS) at rest and during mobilization on postoperative day 1–5. A higher score indicates severe pain. Dots show mean scores for conventional laparoscopic surgery (CLS) (blue), reduced-port laparoscopic surgery (RPLS) (green), single-incision plus one port laparoscopic surgery (SILS + 1) (red), and single-incision laparoscopic surgery (SILS) (purple). *p < 0.05 when comparing RPLS with CLS. + p < 0.05 when comparing SILS + 1 with CLS. $ p < 0.05 when comparing SILS with CLS
The CRP level was lower in the SILS + 1 group than in the CLS and SILS groups at 24 h and 96 h postoperatively (p < 0.05) (Fig. 6A), and the differences were statistically significant at both time points. The IL-6 level was statistically significantly lower in the SILS + 1 group than in the CLS and SILS groups at 24 h postoperatively (p < 0.05; Fig. 6B). No significant differences were observed in WBC and TNF-α levels among the CLS, SILS, and SILS + 1 groups at each time point (Fig. 6C, D).
The median values of C-reactive protein (CRP) A, interleukin-6 (IL-6) B, tumor necrosis factor-α (TNF-α) C, and white blood cell (WBC) D. Whiskers indicate 95 % confidence interval. CLS, conventional laparoscopic surgery; SILS + 1, single-incision plus one port laparoscopic surgery; SILS, single-incision laparoscopic surgery. *p < 0.05
Pathologic and oncologic outcomes
Pathologic and oncologic outcomes were similar between the RPLS and CLS groups (Table 4). There were no loco-regional recurrences in the 2 groups. Distant metastasis was experienced by 9 patients, and the most common site was the liver. All patients were alive at the writing of this article except one in the CLS group, who died of disease progression at 23 months after primary surgery. DFS and OS at 3 years postoperatively were 91.7 versus 89.6 % (p = 0.686) (Fig. 7) and 100.0 versus 97.2 % (p = 0.424) in the RPLS and CLS groups, respectively.
Quality of life
No significant differences were seen among the preoperative and 1-month, 3-month and 6-month postoperative assessments between the RPLS and CLS groups except for social functioning scores at 6 months postoperatively (95.1 ± 9.7 in the RPLS group vs. 90.3 ± 11.3 in the CLS group, p = 0.011) (Table 5, 6 and Supplemental Tables 1 and 2).
Body image and cosmetic assessment
Body Image Questionnaire
For body image and the cosmetic scale, no significant difference was found between the CLS and RPLS groups at anytime point (Supplemental Fig. 1). At 6 months postoperatively, the self-confidence scores were significantly higher than the preoperative scores in the SILS (p = 0.005) and SILS + 1 groups (p = 0.031), but this was not found in the CLS group (p = 0.578) (Supplemental Fig. 2).
Photo series questionnaire
Before seeing the results of alternative approaches, the scores that patients gave to their own scars were similar between the RPLS and CLS groups and between the SILS and SILS + 1 groups at each time point. After viewing the photographs of the alternative approaches, the scores that patients gave to their own scars significantly decreased in the CLS group (from 8.5 ± 1.3 to 7.3 ± 1.1, p < 0.001) but remained similar in the RPLS group. After viewing the photographs, all patients graded the photograph of the CLS scar (6.8 ± 1.3) significantly lower than either the SILS + 1 scar (8.8 ± 1.0, p < 0.001) or the SILS scar (9.0 ± 0.8, p < 0.001), but they chose similar scores for the SILS and SILS + 1 scars (p = 0.297).
When asked their preference of surgical type if they had to undergo the surgery again (Fig. 2, question 6), 46.9 % of all patients preferred SILS; 31.3 % preferred SILS + 1; and 13.5 % preferred CLS. In terms of postoperative morbidity evaluation and management (Fig. 2, question 7), 79.2 % of all patients preferred SILS + 1; 18.8 % preferred CLS; and 2.1 % preferred SILS.
Discussion
Since Bucher et al. performed the first SILS for colorectal diseases, many surgeons have reported their experiences [18]. Multiple studies have shown that the short-term safety and oncological safety of SILS are equal to those of CLS for colorectal cancer, and SILS has the potential to improve both convalescence and cosmetic results [4–7, 19]. However, the widespread clinical use of SILS is seriously impeded by technical difficulties. To minimize the abdominal trauma and technical difficulties, SILS + 1 has gained increasing attention from colorectal surgeons [10, 11]. Many studies have reported that SILS + 1 offers short-term safety results that are comparable to those of CLS [8, 11], but whether SILS + 1 is simple enough for clinical promotion and whether it is as safe as CLS for colorectal cancer while still retaining the minimally invasive benefits of SILS are still unknown. Our institute designed this prospective study to evaluate the safety and benefits of SILS + 1, SILS, and CLS and aimed to answer to the questions presented above.
In our study, the short-term safety and 3-year long-term oncologic safety were comparable between the RPLS and CLS groups, while the RPLS group experienced faster convalescence, less pain, and better cosmetic satisfaction, self-confidence, and social functioning than the CLS group. The SILS + 1 group showed comparable cosmetic results with the SILS group and the lowest inflammatory responses compared to both the SILS and CLS groups. To the best of our knowledge, this is the first study to compare RPLS and CLS for rectosigmoid cancer in terms of quality of life. In addition, this is a pioneering study that thoroughly compared the short- and long-term safety and minimally invasive results among the SILS, SILS + 1, and CLS methods.
Short-term safety
The short-term safety is greatly influenced by the technical difficulties of the procedure. In the present study, the estimated blood loss, morbidity, conversion rate, and operation time were comparable between the RPLS and CLS groups, as many previous studies have reported [6, 7, 11]. When it was difficult to finish the surgery with SILS, adding one additional port allowed a successful finish of the surgery. Katsuno et al. [19] reported that 107 patients underwent SILS, and 8 of those were converted to SILS + 1 because of surgical difficulties and were successfully completed. No conversions to a multiport approach or open surgery were reported. As for operation time, the total time and the procedure time were the shortest in the SILS + 1 group of the present study. This indicates that SILS + 1 may significantly reduce the technical difficulties compared with SILS and may minimize the influence of unskilled assistants in terms of operation time without significantly increasing technical difficulties compared with CLS. Moreover, the additional port used with SILS + 1 was also convenient for drainage. As the total operation time was the shortest in the SILS + 1 group compared to the SILS and CLS groups without jeopardizing short-term safety, the clinical promotion of SILS + 1 is suggested for experienced surgeons.
Minimally invasive results
Pain intensity and postoperative recovery
Postoperative pain is an important factor affecting postoperative recovery. Whether RPLS can reduce postoperative pain remains controversial [4, 6, 7, 19, 20]. To improve the reliability and accuracy of the pain scores, we measured pain intensity (both at rest and during mobilization) until POD 5 and routinely used PCIA until POD 2 in both groups. Interestingly, the pain intensity was significantly less only on POD 3–5 during mobilization in the RPLS group compared to the CLS group, but no significant difference was found at rest or on POD 1–2 during mobilization between the 2 groups. We concluded that compared to CLS, RPLS could reduce postoperative pain during mobilization but not at rest. This may be because the stretching of the wound during mobilization increases pain. However, it is not known why no difference was found between the 2 groups during mobilization on POD 1–2. Theories include the following: (1) The movement of the patients on POD 1–2 was slight, which may not seriously stretch the wound and (2) the routine use of PCIA on POD 1–2 may diminish the differences in pain intensity between the 2 groups. The other postoperative recovery measurements (such as time to liquid diet and ambulation and length of hospital stay) were faster in the RPLS group than in the CLS group, which may be because the patients who underwent RPLS had less postoperative pain, and the belief that they were undergoing minor surgery may have helped them be more willing to resume their normal daily life activities [21].
Inflammatory responses
Surgical trauma induces an inflammatory response and may lead to a transient impairment of the immune system, and therefore is potentially associated with morbidity, local recurrences, and distant metastases [22, 23]. In the present study, no difference was found at anytime point between the SILS and CLS groups, as reported in the previous studies [20, 22]. Interestingly, the CRP levels at 24 and 96 h postoperatively and the IL-6 levels at 24 h postoperatively in the SILS + 1 group were significantly lower than in the SILS and CLS groups. The first possible reason is that the total operative time in the SILS + 1 group was significantly shorter than in the SILS and CLS groups. The second possible reason is the incision length contributes to less surgically induced stress. Therefore, we concluded that the SILS + 1 procedure could reduce surgical stress compared with the SILS and CLS groups.
Cosmesis results
In the present study, we have not only investigated the patients’ satisfaction with their scars and with the alternative approaches but also asked their preference in terms of surgical approaches while taking postoperative safety into consideration. The results indicate that RPLS has better cosmetic results than does CLS, and this could also explain why the self-confidence of patients in the RPLS group improved at 6 months postoperatively. In addition, SILS + 1, which is more convenient for postoperative drainage without reducing the cosmetic results compared with SILS, might be preferred by more patients with rectosigmoid cancer.
Quality of life
In the present study, RPLS did not offer a QOL benefit over CLS in patients with rectosigmoid cancer except for the social functioning score, which may be influenced by scar satisfaction. The elimination of 3–4 trocar incisions could improve convalescence during the hospital stay but not after the 1st month postoperatively.
Oncological safety
Oncological safety is an important measurement for a new surgical technique in the field of radical cancer resection. Three main factors greatly influence oncological outcomes: the quality of the surgical procedure, the pathologic outcomes, and the adjuvant chemotherapy completion rate. To improve the surgery quality control, all surgeries were performed by the same surgeon following the guidelines [24]. To improve the pathologic outcomes, the specimens were examined according to the AJCC guidelines [13] by experienced pathologists. To improve the adjuvant chemotherapy completion rate, we reminded patients to take adjuvant chemotherapy as needed. The RPLS and CLS showed equivalent pathological outcomes and chemotherapy completion rates in our study, so it is not surprising that the oncological outcomes (3-year DFS and OS) were also similar in both groups; these results were also confirmed by the previous studies [6, 7, 19].
The main limitations of this study were as follows: (1) This study was not a randomized study, although the data in our database were prospectively collected; (2) the study was carried out in only one center; and (3) the sample size was relatively small. In this study, the propensity-matched analysis may minimize the selection bias. We are currently conducting a prospective randomized controlled trial with a large sample size to evaluate the comparability and superiority of SILS + 1 compared with CLS (ClinicalTrials.gov NCT02117557).
Conclusions
SILS + 1, which retained the minimally invasive benefits of SILS and significantly reduced the technical difficulties, is a promising alternative approach for rectosigmoid cancer.
Abbreviations
- CLS:
-
Conventional laparoscopic surgery
- RPLS:
-
Reduced-port laparoscopic surgery
- SILS:
-
Single-incision laparoscopic surgery
- SILS + 1:
-
Single-incision plus one port laparoscopic surgery
- QOL:
-
Quality of life
- BMI:
-
Body mass index
- EORTC:
-
European Organization for Research and Treatment of Cancer
- PCIA:
-
Patient-controlled opioid-based intravenous analgesia
- POD:
-
Postoperative day
- WBC:
-
White blood cell
- CRP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- VAS:
-
Visual analogue scale
References
Bonjer HJ (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10:44–52
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AMH, Heath RM, Brown JM (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Curcillo PG 2nd, Podolsky ER, King SA (2011) The road to reduced port surgery: from single big incisions to single small incisions, and beyond. World J Surg 35:1526–1531
Takemasa I, Uemura M, Nishimura J, Mizushima T, Yamamoto H, Ikeda M, Sekimoto M, Doki Y, Mori M (2014) Feasibility of single-site laparoscopic colectomy with complete mesocolic excision for colon cancer: a prospective case-control comparison. Surg Endosc 28:1110–1118
Kim SJ, Ryu GO, Choi BJ, Kim JG, Lee KJ, Lee SC, Oh ST (2011) The short-term outcomes of conventional and single-port laparoscopic surgery for colorectal cancer. Ann Surg 254:933–940
Kim CW, Cho MS, Baek SJ, Hur H, Min BS, Kang J, Baik SH, Lee KY, Kim NK (2015) Oncologic outcomes of single-incision versus conventional laparoscopic anterior resection for sigmoid colon cancer: a propensity-score matching analysis. Ann Surg Oncol 22:924–930
Yun JA, Yun SH, Park YA, Huh JW, Cho YB, Kim HC, Lee WY (2016) Oncologic outcomes of single-incision laparoscopic surgery compared with conventional laparoscopy for colon cancer. Ann Surg. 263:973–978
Lim SW, Kim HJ, Kim CH, Huh JW, Kim YJ, Kim HR (2013) Umbilical incision laparoscopic colectomy with one additional port for colorectal cancer. Tech Coloproctol 17:193–199
Gash K, Bicsak M, Dixon A (2015) Single incision laparoscopic surgery for rectal cancer: early results and medium term oncological outcome. Colorectal Dis 17:1071–1078
Hamabe A, Takemasa I, Uemura M, Nishimura J, Mizushima T, Ikeda M, Yamamoto H, Sekimoto M, Doki Y, Mori M (2014) Feasibility of single-port laparoscopic surgery for sigmoid colon and rectal cancers and preoperative assessment of operative difficulty. J Gastrointest Surg 18:977–985
Kawamata F, Homma S, Minagawa N, Kawamura H, Takahashi N, Taketomi A (2014) Comparison of single-incision plus one additional port laparoscopy-assisted anterior resection with conventional laparoscopy-assisted anterior resection for rectal cancer. World J Surg 38:2716–2723
Yu H, Shin JY (2016) Short-term outcomes following reduced-port, single-port, and multi-port laparoscopic surgery for colon cancer: tailored laparoscopic approaches based on tumor size and nodal status. Int J Colorectal Dis 31:115–122
Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti A (eds) (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Whistance RN, Conroy T, Chie W, Costantini A, Sezer O, Koller M, Johnson CD, Pilkington SA, Arraras J, Ben-Josef E, Pullyblank AM, Fayers P, Blazeby JM (2009) Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 45:3017–3026
Dunker MS, Stiggelbout AM, van Hogezand RA, Ringers J, Griffioen G, Bemelman WA (1998) Cosmesis and body image after laparoscopic-assisted and open ileocolic resection for Crohn’s disease. Surg Endosc 12:1334–1340
Fayers P, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A (2001) The EORTC QLQ-C30 scoring manual, 3rd edn. European Organisation for Research and Treatment of Cancer, Brussels
Bucher P, Pugin F, Morel P (2008) Single port access laparoscopic right hemicolectomy. Int J Colorectal Dis 23:1013–1016
Katsuno G, Fukunaga M, Nagakari K, Yoshikawa S, Azuma D, Kohama S (2016) Short-term and long-term outcomes of single-incision versus multi-incision laparoscopic resection for colorectal cancer: a propensity-score-matched analysis of 214 cases. Surg Endosc 30:1317–1325
Hiraki M, Takemasa I, Uemura M, Haraguchi N, Nishimura J, Hata T, Mizushima T, Yamamoto H, Doki Y, Mori M (2014) Evaluation of invasiveness in single-site laparoscopic colectomy, using “the PainVision system” for quantitative analysis of pain sensation. Surg Endosc 28:3216–3223
Moraca RJ, Sheldon DG, Thirlby RC (2003) The role of epidural anesthesia and analgesia in surgical practice. Ann Surg 238:663–673
Bulut O, Aslak KK, Levic K, Nielsen CB, Romer E, Sorensen S, Christensen IJ, Nielsen HJ (2015) A randomized pilot study on single-port versus conventional laparoscopic rectal surgery: effects on postoperative pain and the stress response to surgery. Tech Coloproctol 19:11–22
Jung IK, Kim MC, Kim KH, Kwak JY, Jung GJ, Kim HH (2008) Cellular and peritoneal immune response after radical laparoscopy-assisted and open gastrectomy for gastric cancer. J Surg Oncol 98:54–59
Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D (2001) Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 93:583–596
Acknowledgments
This study was supported by Major Program of Science and Technology Program of Guangzhou (No. 201300000087 and No. 201508020047), Research Fund of Public welfare in Health Industry of National Health and Family Planning Commission of China (No.201402015 and No. 201502039), National Key Technology R&D Program (No.2013BAI05B05), and Key Clinical Specialty Discipline Construction Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
Drs. Ruoyan Liu, Yanan Wang, Ze Zhang, Tingting Li, Hao Liu, Liying Zhao, Haijun Deng, and Guoxin Li have no conflicts of interest or financial ties to disclose.
Additional information
Ruoyan Liu and Yanan Wang have contributed equally to this work and should be considered co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, R., Wang, Y., Zhang, Z. et al. Assessment of treatment options for rectosigmoid cancer: single-incision plus one port laparoscopic surgery, single-incision laparoscopic surgery, and conventional laparoscopic surgery. Surg Endosc 31, 2437–2450 (2017). https://doi.org/10.1007/s00464-016-5244-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5244-8