Abstract
Background
There is currently a paucity of research comparing the clinical outcomes of single-incision laparoscopic colectomy (SILC) with those obtained with multiport laparoscopic colectomy (MLC). This meta-analysis aimed to examine whether SILC shows real benefits over MLC, especially in terms of feasibility, safety, and oncological adequacy.
Methods
A literature review of studies comparing SILC and MLC has been performed which looked at the following outcomes: mortality, morbidity, and oncological parameters of adequacy, as well as other potential benefits and drawbacks. Standardized mean difference for continuous variables and odds ratios for qualitative variables were calculated.
Results
Thirty studies comparing SILC and MLC were reviewed: two prospective randomized clinical trials (RCTs), eight prospective studies, and 20 retrospective comparative observational studies. Overall, in a cohort of 3502 patients who underwent surgery, SILC was used in 1068 cases (30.5 %) and MLC was used in 2434 cases (69.5 %). Mean intraoperative blood loss was significantly lower when the SILC procedure had been used (75.06 vs. 91.45 ml, P = 0.03); bowel function recovered significantly earlier in the SILC patients (1.96 vs. 2.15 days, P = 0.03); mean postoperative hospital stay was significantly shorter in the SILC group (5.55 vs. 6.60 days, P = 0.0005); and length of skin incision was significantly shorter in SILC patients (3.98 vs. 5.28 cm, P = 0.01). However, in the latter four outcomes, evidence of heterogeneity was found. In contrast, MLC showed significantly better results when compared to SILC in terms of distal free margins (12.26 vs. 10.98 cm, P = 0.01).
Conclusions
SILC could be considered as a safe and feasible alternative to MLC in experienced hands. Further evidence for this surgical procedure should be assessed in the form of high-quality RCTs, with additional focus on its use in low rectal cancer resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Multiport laparoscopic colectomy (MLC) is widely accepted among surgeons as it has several advantages: a smaller incision, reduced postoperative pain, shorter hospital stay, faster return to normal activities, and improved cosmetic results when compared to the conventional open approach [1]. The oncological outcomes of MLC are also comparable with those obtained using traditional laparotomy [2].
During the 1990s, single-incision laparoscopic surgery (SILS) was used for the removal of the uterus, gallbladder, and appendix [3]. Subsequently, in 2008 two reports were published describing the preliminary use of SILS in colorectal surgery [4, 5].
The actual benefits of single-incision laparoscopic colectomy (SILC) would presumably include those of MLC, together with reduced surgical trauma, improved cosmetic results, and patient satisfaction. SILC is now used for the treatment of both benign and malignant colorectal diseases. However, the results of the SILC procedure and subsequent oncological outcomes are matters for debate. In fact, there is a paucity of research comparing the clinical outcomes of SILC with those obtained using MLC.
From 2010 onward, 30 studies comparing the clinical outcomes of SILC versus MLC have been published [6–35]. Of these studies, two were prospective, randomized trials [15, 18] and 28 were comparative prospective or retrospective observational studies [6–14, 16, 17, 19–35]. Thus far, four meta-analyses have been performed to compare the results of SILC with those obtained using MLC [36–39]. The majority of these studies were limited by a single-institution design or by small sample size. To overcome these limitations, we performed a new systematic review with meta-analysis, which included the largest number of adult patients from all comparative studies in the literature. We examined whether SILC has an advantage over MLC, in terms of feasibility, safety, and oncological adequacy. Furthermore, we aimed to verify other potential benefits and drawbacks of the technique.
Materials and methods
In this study, SILC was defined as a standardized operation performed through a single abdominal incision at the level of the umbilicus or in other abdominal regions, depending on author preference and type of colonic resection. MLC was defined as a classical laparoscopic technique, performed with three or four trocars or using a hand-assisted procedure, as similar clinical outcomes were shown in trials comparing MLC with hand-assisted technique [40, 41]. Studies comparing the characteristics and perioperative outcomes of adult patients undergoing SILC and MLC for colorectal disease met the inclusion criteria. Prospective, randomized clinical trials or prospective or retrospective observational studies comparing the two techniques were also included in the analysis. Included studies had to be written in English. Studies were excluded from the meta-analysis if the outcomes of interest (as specified below) were impossible to calculate or the standard deviation and confidence interval of the tested parameters were not reported.
A systematic literature search was performed using EMBASE, Medline, Cochrane, PubMed, and Google Scholar databases for studies comparing SILC to MLC. The following keywords: “single-incision laparoscopic colectomy” or “SILC” and “multiport laparoscopic colectomy” or “MLC” were used as search terms. The search was then extended by using the “related article” function of each database and by scanning the references of all relevant articles. The final literature search was completed in March 2015.
The meta-analysis was performed in accordance with the recommendations from the preferred items for systematic reviews and meta-analyses statement (PRISMA) [42], and the meta-analysis of observational studies in epidemiology checklist for observational studies [43].
Two authors (MP and AP) independently extracted the following data from each study: institution and year of publication, study type, the number of patients operated on with each technique, and the baseline characteristics of patients, such as age and gender, perioperative outcomes, and postoperative results.
All included studies were reviewed for the following outcomes of interest:
-
We evaluated the primary outcome measures to assess and validate safety, feasibility, and oncological efficacy of the SILC procedure. The following outcomes were reviewed: mortality and morbidity such as abdominal abscess, postoperative hematoma, wound infection, anastomotic bleeding, and anastomotic leak. Oncological outcomes reviewed: positive margins, tumor diameter, proximal and distal free margins, harvested lymph nodes, and carcinoma recurrence.
-
We evaluated the secondary outcome measures to assess other potential benefits and drawbacks of SILC. The following outcomes were reviewed: previous abdominal surgery and operative outcomes such as operative time, conversion to laparotomy, intraoperative blood loss, reoperation, recovery of bowel function, readmission, length of postoperative hospital stay, length of skin incision, and incisional hernia.
The surgical indication, type of operation, and different surgical methods used for the SILC procedure were also reviewed by the authors.
Statistical analysis, synthesis, and reporting of the results
We considered variables for pooled analysis if they were previously evaluated by at least three studies. We carried out all statistical analyses using Reviewer Manager software (Review Manager—RevMan—version 5.3.5, 2014, The Nordic Cochrane Centre, Cochrane Collaboration, www.cochrane-handbook.org). The meta-analysis was conducted by searching for a numerical estimate of the outcome of interest, as described elsewhere [44]. For continuous outcomes, the Hedges’ g was used for the calculation of the standardized mean difference (SMD) under the fixed-effects model, which we adjusted for small sample bias. Under the fixed-effects model, we assumed that all studies were homogeneous. We tested this assumption using the heterogeneity test, which we included to calculate the summary SMD under the random-effects model, according to the method of DerSimonian and Laird [45]. We tested for heterogeneity using the random-effects model when calculating the Chi2 test and its associated P values. If this test yielded a P value <0.05, then the fixed-effects model was considered as invalid and the random-effects model as appropriate. We listed the results of the individual studies and gave the total SMD with a 95 % confidence interval (CI) for both the fixed-effects model and the random-effects model. If the value of 0 was not within the 95 % CI, then we considered the SMD statistically significant at the 5 % level (P < 0.05). The heterogeneity was also tested using the I 2 test. I 2 is the percentage of observed total variation across studies that is due to real heterogeneity, rather than chance. It is calculated as I 2 = 100 % × (Q − df)/Q, where Q is Cochran’s heterogeneity statistic and df the degrees of freedom. Negative values of I 2 are considered equal to zero, so that I 2 lies between 0 % and 100 %. A value of 0 % indicates no observed heterogeneity, and larger values show increasing heterogeneity [46]. This method required the standard deviations and the confidence intervals of the tested parameters. The results of different studies were summarized and reported using a forest plot with a 95 % CI and overall SMD.
For data derived from contingency tables (qualitative outcomes), the odds ratio (OR) and 95 % CI were calculated. The ORs reported in the results are those of the pooled analysis method, also called pooled ORs. We used the Mantel–Haenszel method for calculating the weighted summary OR under the fixed-effects model and then incorporated the heterogeneity test to calculate the summary OR under the random-effects model, according to the method of DerSimonian and Laird [45]. If this test yielded a P value <0.05, then we considered the fixed-effects model as invalid and the random-effects model as appropriate. The heterogeneity was also tested using the I 2 test [46]. We have listed the results of individual studies and have given the total OR with 95 % CI for both the fixed-effects model and the random-effects model. If the value 1 was not within the 95 % CI, then we considered the OR to be statistically significant at the 5 % level (P < 0.05). We summarized the results of different studies, with 95 % CI, and the overall effect (summary OR), with 95 % CI, on a logarithmic scale using a forest plot.
Results
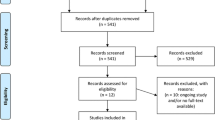
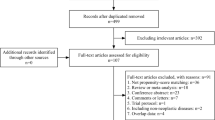
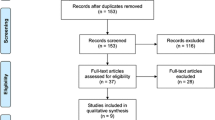
The PRISMA flowchart for systematic search and selection of articles for review and meta-analysis is shown in Fig. 1. We considered 30 studies comparing colorectal resections with SILC versus MLC as suitable for the pooled analysis [6–35]. Two comparative studies were excluded because there was a concern regarding duplication of data [47, 48]. The articles included in the quantitative synthesis were published between 2010 and 2015. Of these studies, ten had been conducted in the USA, four in Korea, three in Japan, two in Italy, two in Taiwan, two in the UK, two in France, one in Belgium, one in Hong Kong, one in Singapore, one in Australia, and one in the Netherlands. They included a total of 3502 patients with colon resections performed using SILC in 1068 (30.5 %) and MLC in 2434 (69.5 %) patients, respectively. In two prospective, randomized clinical trials (RCTs), the patients had been randomly assigned to either SILC or MLC groups [15, 18]. The other investigations included were eight prospective [22–25, 29–31, 35] and 20 retrospective comparative observational studies [6–14, 16, 17, 19–21, 26–28, 32–34]. The study by Katsuno et al. [16] was a poster presented at the 2012 Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES).
The mean follow-up period was 15 months (range 3–35 months), as reported in the included studies [10, 15, 20, 25, 28, 29]. The two reviewers were in agreement regarding the data extracted from the studies. The characteristics of the studies, including the demographics of the included patients, indication for surgery and type of operation, and different methods used for SILC, are given in Tables 1 and 2.
The types of operation performed in the SILC group were: right hemicolectomy (586), anterior resection (171), low anterior resection (24), transverse colectomy (10), left colectomy (96), subtotal colectomy (3), sigmoidectomy (80), ileocolonic resection (46), ileocecal resection (9), total colectomy (6), proctocolectomy (17) and trans-abdominal trans-anal resection (20). The types of operation performed in the MLC group were: right hemicolectomy (799), anterior resection (583), low anterior resection (193), ileocolic resection (116), transverse colectomy (13), left colectomy (283), total colectomy (135), sigmoidectomy (55), proctectomy with TME (165), proctectomy without TME (44), Hartmann procedure (1), ileocecal resection (7), and proctocolectomy (40), as given in Table 1.
Primary outcome measures
Outcomes evaluated to assess feasibility and safety of SILC: mortality, overall morbidity, abdominal abscess, postoperative hematoma, wound infection, anastomotic bleeding, and anastomotic leak.
Mortality rate was similar in both groups, without a statistically significant difference (0.0028 vs. 0.0065, P = 0.62, OR 0.79, 95 % CI 0.31–2.01, for SILC and MLC, respectively; no heterogeneity was found: P = 0.98, I 2: 0 %, Fig. 2; Table 3). One patient in the SILC group died from significant comorbidities in the postoperative period [20], a second patient died of pulmonary embolus [30], and a third patient died of neutropenic sepsis secondary to a Gram-negative urinary tract infection [33]. A total of 16 patients in the MLC group died during the postoperative period: one from a cerebrovascular accident, one from severe pneumosepsis [20], two from respiratory complications [27], two from myocardial infarction [6, 32], and ten died due to unspecified reasons [17, 23, 35].
In the meta-analysis of studies comparing overall morbidity rates after SILC (13.20 %) and MLC (13.06 %), there was no significant difference (0.132 vs. 0.130, P = 0.15, OR 0.84, 95 % CI 0.67–1.06; no heterogeneity was found: P = 0.94, I 2: 0 %, Fig. 3; Table 3).
Abdominal abscess occurred in three patients (0.28 %) in the SILC group and in four patients (0.16 %) in the MLC group, but this difference was not significant (0.0028 vs. 0.0016, P = 0.91, OR 0.92, 95 % CI 0.24–3.52; no heterogeneity was found: P = 0.73, I 2: 0 %, Fig. 4; Table 3).
The prevalence of postoperative hematoma was similar in both groups, being 0.37 % in the SILC group and 0.32 % in the MLC group (0.0037 vs. 0.0032, P = 0.89, OR 0.93, 95 % CI 0.34–2.55; no heterogeneity was found: P = 0.75, I 2: 0 %, Fig. 4; Table 3).
The prevalence of wound infection was slightly higher in the SILC group (2.24 %) than in the MLC group (1.15 %), but this difference was not significant (0.0224 vs. 0.0115, P = 0.53, OR 1.18, 95 % CI 0.70–2.00; no heterogeneity was found: P = 0.98, I 2: 0 %, Fig. 4; Table 3).
Anastomotic bleeding was shown in 1.40 % of SILC procedures and in 0.24 % of MLC procedures, but this difference was not significant (0.0140 vs. 0.0024, P = 0.10, OR 2.56, 95 % CI 0.85–7.74; no heterogeneity was found: P = 0.45, I 2: 0 %, Fig. 5; Table 3).
The prevalence of anastomotic leak was slightly lower in the SILC group (1.87 %) than in the MLC group (4.31 %), but the difference was not significant (0.0187 vs. 0.0431, P = 0.42, OR 0.82, 95 % CI 0.51–1.33; no heterogeneity was found: P = 0.95, I 2: 0 %, Fig. 5; Table 3). Anastomotic leak occured following anterior resection in both groups. However, the number of low anterior resections in SILC colectomies was just 24 out of 195 (12.3 %) compared with 193 out of 776 (24.9 %) in the MLC group. This difference was statistically significant (P = 0.01, by χ 2 test). Moreover, the number of right colectomies performed in the SILC group was significantly higher than the MLC group (0.56 vs. 0.41, P = 0.000, by χ 2 test).
Outcomes evaluated to assess oncological efficacy of SILC: positive margins, proximal and distal free margins, harvested lymph nodes, and carcinoma recurrence.
It was impossible to meta-analyze the outcome regarding positive margins after colonic resection because all authors reported negative margins. There was no statistically significant difference between SILC and MLC groups regarding tumor diameter (3.43 vs. 3.56 cm, P = 0.85, SMD = 0.01, 95 % CI −0.13 to 0.16; no heterogeneity was found: P = 0.067, I 2: 46 %, Table 4). Average proximal free margin from the tumor was similar in both the SILC group and the MLC group (13.01 vs. 11.35 cm, P = 0.53, SMD = 0.09, 95 % CI −0.19 to 0.37; heterogeneity was found: P = 0.01, I 2: 66 %, Fig. 6; Table 4). However, average distal free margin from the tumor was significantly longer in the MLC group than in the SILC group (12.26 vs. 10.98 cm, P = 0.01, SMD = 0.19, 95 % CI 0.04–0.35; no heterogeneity was found: P = 0.14, I 2: 38 %, Fig. 6; Table 4). The number of harvested lymph nodes was similar in the SILC group and in the MLC group (18.59 vs. 18.82 lymph nodes, P = 0.23, SMD = 0.11, 95 % CI −0.07 to 0.28; heterogeneity was found: P = 0.01, I 2: 53 %, Fig. 6; Table 4). Only three studies reported results regarding tumor recurrence, with a mean follow-up of 15 months. The lack of further follow-up data made comprehensive meta-analysis of this outcome impossible.
Secondary outcome measures
Outcomes evaluated to assess other potential benefits and drawbacks of SILC: body mass index (BMI), previous abdominal surgery, operative time, conversion to laparotomy, intraoperative blood loss, reoperation, recovery of bowel functions, readmission, length of postoperative hospital stay, and length of skin incision.
The BMI was significantly lower in the SILC group compared with the MLC group (24.86 vs. 25.67 kg/m2, P = 0.04, SMD = −0.22, 95 % CI −0.43 to −0.001; heterogeneity was found: P < 0.0001, I 2: 71 %, Table 1).
Previous abdominal surgery was not a contraindication when performing both SILC and MLC procedures. A previous abdominal operation was performed in 14.23 % of patients in the SILC group versus 14.50 % of patients in the MLC group. This difference was not significant (P = 0.12, OR 0.82, 95 % CI 0.65–1.05; no heterogeneity was found: P = 0.93, I 2: 0 %, Table 5). A history of previous abdominal operations was not always reported in the included studies.
No statistically significant difference was found in the meta-analysis of studies comparing SILC and MLC for operative time (147.28 vs. 148.97 min, P = 0.58, SMD = 0.09, 95 % CI −0.22 to 0.39; heterogeneity was found: P < 0.00001, I 2: 85 %, Fig. 7; Table 5).
The rate of conversion to laparotomy was lower in the SILC group (1.40 %) than in the MLC group (3.12 %), but this difference was not significant (0.0140 vs. 0.0312, P = 0.11, OR 0.64, 95 % CI 0.38–1.10; no heterogeneity was found: P = 0.91, I 2: 0 %, Fig. 8; Table 5). The reasons for conversion to open surgery in the SILC group were: two large tumors, two dense adhesions, one bulky omentum, one case of dense retroperitoneal fibrosis, one case of inability to identify left ureter, one case of ileocolic artery bleeding, one mesenteric tearing, one difficult splenic flexure mobilization, two cases of severe inflammation, and one case of intraoperative colonic injury. The reasons for conversion to open surgery in the MLC group were: six adhesions, one case of inability to visualize the tattoo, one case of poor visibility for thick omentum, one dilated proximal bowel, one large tumor, one diverticular abscess, one case of anatomical difficulties, one bleeding of the inferior mesenteric artery, one case of inability to laparoscopically separate the left ureter from an abscess, one case of intraoperative vascular complications, and 61 unspecified cases. According to the most part of authors, we considered conversion from SILC to MLC, even if only one additional trocar was used. The reasons for conversion from SILC to MLC were: three cases of difficult exposure of the peritoneal reflection, 14 cases of difficult pelvic wall dissection, six adhesions, one case of bleeding of the gonadic artery, one discovery of a bulky tumor with presacral fixation, one dense pelvic abscess cavity, one redundant sigmoid colon, one case of inadequate colonic traction, one friable Crohn’s mesentery, one unclear anatomy, and 34 unspecified cases.
Mean intraoperative blood loss was significantly lower in the SILC group than in the MLC group (75.06 vs. 91.45 ml, P = 0.03, SMD = −0.26, 95 % CI −0.48 to −0.03; heterogeneity was found: P = 0.03, I 2: 53 %, Fig. 7; Table 5).
The reoperation rate was slightly lower in the SILC group (1.38 %) than in the MLC group (2.75 %), but not significantly (0.0138 vs. 0.0275, P = 0.50, OR 0.79, 95 % CI 0.40–1.56; no heterogeneity was found: P = 0.78, I 2: 0 %, Fig. 9; Table 5). The reasons for reoperation were: anastomotic leak, postoperative bleeding, intraabdominal abscess, fascial dehiscence, perforation for cecal ischemia, and thermal injury of the transverse colon.
Bowel function recovered significantly earlier in terms of flatus in the SILC group compared with the MLC group (1.96 vs. 2.15 days, P = 0.03, SMD = −0.28, 95 % CI −0.53 to −0.03; heterogeneity was found: P = 0.04, I 2: 59 %, Fig. 10; Table 6). We did not meta-analyze data regarding starting a postoperative regular diet because only two studies reported mean and standard deviation [7, 23].
The rate of readmission was moderately lower in the SILC group (0.93 %) than in the MLC group (1.23 %), but not significantly (0.0093 vs. 0.0123, P = 0.83, OR 0.92, 95 % CI 0.44–1.93; no heterogeneity was found: P = 0.49, I 2: 0 %, Table 6). The reasons for readmission were ileus, anastomotic leak, abdominal hematoma, abdominal abscess, wound infection, and stroke-like symptoms.
Mean postoperative hospital stay was significantly shorter in the SILC group than in the MLC group (5.55 vs. 6.60 days, P = 0.0005, SMD = −0.27, 95 % CI −0.42 to −0.12; heterogeneity was found: P = 0.02, I 2: 47 %, Fig. 7; Table 3).
Length of skin incision was significantly shorter in the SILC group than in the MLC group (3.98 vs. 5.28 cm, P = 0.01, SMD = −0.94, 95 % CI −1.65 to −0.22; heterogeneity was found: P < 0.00001, I 2: 93 %, Fig. 10; Table 5).
Subgroup analysis: right hemicolectomies
Complications were reported for 16.7 % of patients who underwent a right hemicolectomy in the SILC group and for 15.3 % of patients in the MLC group, without any statistically significant difference (OR 1.06, 95 % CI 0.70–1.59, P = 0.79; no heterogeneity was found for I 2 = 0 %; P = 0.82). No significant difference was reported for operative time (SMD, −0.19, 95 % CI −0.48 to 0.10, P = 0.20; no heterogeneity was found for I 2 = 20 %; P = 0.26), and postoperative hospital stay (SMD, −0.19, 95 % CI −0.47 to 0.10, P = 0.20; no heterogeneity was found for I 2 = 0 %; P = 0.66).
Subgroup analysis: anterior resections
Complications were reported for 7.6 % of patients who underwent an anterior resection in the SILC group and for 4.5 % of patients in the MLC group, without any significant difference (OR 1.52, 95 % CI 0.57–4.05, P = 0.40; no heterogeneity was found for I 2 = 0 %; P = 0.55). No significant difference was reported for operative time (SMD, −0.27, 95 % CI −1.32 to 0.77, P = 0.61; heterogeneity was found for I 2 = 93 %; P = 0.0003) and conversion to laparotomy, the rate being 1.3 % in the SILC group and 3.3 % in the MLC group (OR 0.56, 95 % CI 0.21–1.54, P = 0.26; no heterogeneity was found for I 2 = 0 %; P = 0.73).
Subgroup analysis: studies including low anterior resections
Two retrospective cohort studies [23, 34] reported outcomes for patients who underwent a low anterior resection (LAR). LAR represented only 20.6 % of the total number of procedures analyzed in the SILC group and 49.6 % of those in the MLC group. Morbidity rate was similar when comparing the two techniques: 25.6 % of cases in the SILC group and 23.1 % cases in the MLC (OR 0.82, 95 % CI 0.49–1.38, P = 0.46; no heterogeneity was found for I 2 = 0 %; P = 0.82). No significant difference was found when analyzing the outcomes: postoperative hospital stay (SMD, −0.27, 95 % CI −0.83 to 0.08, P = 0.11; heterogeneity was found for I 2 = 76 %; P = 0.04), and bowel function recovery (SMD, −0.22, 95 % CI −0.65 to 0.21, P = 0.31; heterogeneity was found for I 2 = 74 %; P = 0.05). Conversely, the length of the distal free margin was significantly longer in the MLC group (SMD 1.78, 95 % CI 0.18 to 3.39, P = 0.03; no heterogeneity was found for I 2 = 59 %; P = 0.12). The mean intraoperative blood loss was impossible to meta-analyze for this subgroup of patients.
Publication bias
Funnel plots demonstrated moderate asymmetry for operative time and length of skin incision, suggesting the possibility of publication bias for these outcomes (Fig. 11). No points fell outside of the 95 % confidence interval limits for any other outcome of interest, suggesting the absence of publication bias.
Discussion
There has been a surgical evolution from open to conventional laparoscopic colorectal surgery for treatment of both benign and malignant diseases in most tertiary referral centers. In recent years, SILC has been an attractive and fascinating technique for surgeons willing to further improve laparoscopic operations. However, the role of the single-incision approach in colorectal surgery is still a matter of debate, as no conclusive data exist regarding short-term and long-term outcomes of the procedure.
The outcome measures of our systematic review with meta-analysis showed that there was no statistically significant difference in terms of mortality, overall and specific morbidity, and operative time when comparing the SILC patient group with the MLC patient group.
Likewise, when evaluating the oncological adequacy of SILC, the results were similar in both SILC and MLC groups. Four of the secondary outcomes significantly favored the SILC group over the MLC group: Mean intraoperative blood loss was significantly lower using the SILC procedure (75.06 vs. 91.45 ml, P = 0.03); bowel function recovered significantly earlier in the SILC patients (1.96 vs. 2.15, P = 0.03); mean postoperative hospital stay was significantly shorter in the SILC group than in the MLC group (5.55 vs. 6.60, P = 0.0005); and length of skin incision was significantly shorter in the SILC group than in the MLC patients (3.98 vs. 5.28, P = 0.01). In contrast, our meta-analysis demonstrated that the MLC was superior to the SILC procedure for length of the distal free margin (12.26 vs. 10.98 cm, P = 0.01).
Our results confirm the hypothesis in much of the current literature that SILC is safe and feasible for the treatment of benign and malignant colorectal diseases, with short-term results comparable to that of MLC. When calculating the primary outcome measures for our research, no heterogeneity was found across the included studies for mortality rate, overall morbidity, and specific complications. In contrast, the proximal and distal free margin, and harvested lymph nodes, showed a degree of heterogeneity. Conversely, specific postoperative results included in the secondary outcomes were significantly better in the SILC group than in the MLC group, even when evidence of heterogeneity was observed.
To the best of our knowledge, this is the fifth meta-analysis to compare the results of SILC versus MLC in colorectal surgery. Previous pooled analyses were based on 27 comparative studies and one randomized controlled trial [39], 15 comparative studies [36, 37], or 11 comparative studies [38]. The results of our meta-analysis, based on a total of 3502 laparoscopic procedures, included the largest number of adult patients from 30 comparative studies in the literature.
The need for timely summarized data regarding important clinical questions also justifies the use of pooled analysis, including observational studies, when there is a lack of randomized controlled trials. However, meta-analyses based on observational studies are more prone to bias, resulting in low-quality evidence [43].
With the exception of two RCTs [15, 18], 17 of 27 of the comparative studies included were designed as case-matched studies [6, 7, 9, 12–14, 16, 19–22, 25, 28–31, 35]. Pooling matched case–control design studies should reduce the confounding effects of covariates on the treatment results [13].
The majority of patients in this meta-analysis were oncology patients. The parameters used to evaluate the oncological appropriateness of the SILC procedure, such as the number of harvested lymph nodes and resection margins, were adequate in all studies included. However, there were no data regarding long-term outcomes, such as disease-free and cancer-related survival, as all the studies included were published between 2010 and 2015. For SILC to be retained as a standard procedure for the treatment of colorectal cancer, it should also be supported by favorable long-term oncological results [23].
Among the secondary outcomes that significantly favored SILC, blood loss without blood transfusion and earlier recovery of bowel function are not likely to be of clinical significance, as other authors have also reported [47]. Despite mean postoperative hospital stay being significantly shorter in the SILC than in the MLC group (5.5 vs. 6.6 days, P = 0.005), a previously published series of conventional laparoscopic colon resection have reported a median hospital stay of 4 or 5 days [49]. The length of skin incision was significantly shorter in the SILC than in the MLC group (3.98 vs. 5.28 cm, P = 0.01). However, a bigger specimen size required a larger incision for the extraction, and thus, some authors have suggested measuring the length of the incision at the end of the operation, rather than at the beginning [50, 51]. One of the hypothetical benefits of SILC should be improved cosmesis and patient satisfaction, which are related to the final length of the skin incision. This outcome was impossible to meta-analyze because only Lee et al. [25] addressed the issue, finding cosmetic scores higher for SILC than for MLC, without any difference in body image score. Moreover, drawing a conclusion regarding cosmetics results is not easy, as satisfaction score may be age- and gender-related, and could be irrelevant in elderly patients undergoing operations for cancer [20].
It is important to note that the number of low anterior resections performed in the SILC group was significantly lower than in the MLC group. SILC patients underwent right hemicolectomy in 56 % of cases (599 patients), whereas the percentage of low anterior resections performed in this group was only 12 % (128 patients). Conversely, patients in the MLC group underwent right hemicolectomy in 41 % of cases (998 patients), whereas a low anterior resection was performed in 25 % (609 patients).
Moreover, in our study the mean BMI of surgical patients was 24.86 in the SILC group versus 25.67 in the MLC group, with a statistically significant difference (P = 0.04). Data were insufficient to allow meaningful conclusions to be drawn regarding obese patients with a BMI > 30 kg/m2. In a recent case-matched study, primarily based on patients who underwent SILC for benign disease, Keller et al. reported that SILC in obese patients had significantly longer operative times and higher blood loss, but comparable conversion rates, oncologic outcomes, lengths of stay, complication, and readmission rates as the non-obese cohorts [52].
As in colorectal surgery obesity poses additional technical challenges, safety and feasibility of SILS in obese patients remains one of the most important issues for clarification in the future.
Moreover, these results point out a selection bias which may have influenced the outcomes, as the most complex operations, and probably the most “difficult patients” have been approached by MLC.
Postoperative pain score evaluation was impossible to meta-analyze because few studies addressed this issue. In the RCT by Poon et al. [18], postoperative pain score evaluated by the visual analog scale was significantly lower in the SILC group than in the MLC group, which subsequently reduced hospital stay in the SILC group. Conversely, greater wound irritation, due to insertion of all surgical instruments through a single incision, may increase the intensity of postoperative pain sensation [53]. The true evaluation of postoperative pain score after SILC procedure will be sufficiently assessed by prospective RCTs only.
Data from pooled studies were insufficient to define a learning curve after which laparoscopic surgeons can safely master SILC. In the beginning, difficulties with instruments overcrowding and triangulation can make the SILC procedure cumbersome [22, 50]. An experienced and well-trained laparoscopic surgeon will also subsequently overcome these disadvantages by the use of new and innovative instruments [29, 50].
Only three of the included studies reported results relating to the operative costs of SILC, but standard deviations were provided only by Fujii et al. [13]. Therefore, the meta-analysis for this outcome of interest could not be carried out. However, Fujii reported a statistically significant difference in the cost of access instruments between the two groups. The total mean per-patient cost of access instruments was 62.761 ± 2.946 Japanese yen with SILC and 77.130 ± 7.869 Japanese yen with MLC [13]. McNally et al. [27] reported that additional cost for SILC was approximately 250 American dollar. In a study published by Waters et al. [32], the marginal increase in direct operative cost for SILC was 310–410 American dollars per case. However, the single-incision technique can reduce the number of traditional trocars used, thereby minimizing the cost gap. This data showed that the additional cost per-patient was always related to the major cost of the particular single-port used. Thus, SILC should be more cost-effective than MLC when a statistically significant difference in improved postoperative recovery is demonstrated by RCTs.
Finally, a comprehensive analysis of the outcomes of interest for our research shows that the expectation of benefits using the SILC procedure must not compromise patient safety. The majority of the included studies were conducted in highly specialized laparoscopic units, and this may be a limiting factor for the extensive use of the SILC procedure in less specialized units where required expertise is unavailable [17].
In conclusion, SILC could be considered as a safe and feasible alternative to MLC in experienced hands, and in selected patients. However, due to the very small number of single-incision low anterior resections analyzed in this systematic review, a clear indication for low rectal cancer cannot be validated. Moreover, the statistically significant lower BMI reported in the SILC group suggests the presence of selection biases within current research, which was primarily based on data from observational studies. Therefore, the results must be approached with caution. Before recommending SILC for everyday clinical practice in colorectal surgery, we believe that all aspects of the procedure should be better assessed by high-quality multicenter prospective RCTs and subsequent clustered meta-analysis, with special regard to low rectal cancers.
References
Wexner SD, Reissman P, Pfeifer J, Bernstein M, Gerome N (1996) Laparoscopic colorectal surgery: analysis of 140 cases. Surg Endosc 10:133–136
Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ (2008) Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. doi:10.1002/14651858.CD003432.pub2
Fung AKY, Aly EH (2012) Systematic review of single-incision laparoscopic colonic surgery. Br J Surg 99:1353–1364
Remzi FH, Kirat HT, Kaouk JH, Geisler DP (2008) Single-port laparoscopy in colorectal surgery. Colorectal Dis 10:823–826
Bucher P, Pugin F, Morel P (2008) Single port access laparoscopic right hemicolectomy. Int J Colorectal Dis 23:1013–1016
Chew M-H, Chang M-H, Tan W-S, Wong M-T, Tang C-L (2013) Conventional laparoscopic versus single-incision laparoscopic right hemicolectomy: a case cohort comparison of short-term outcomes in 144 consecutives cases. Surg Endosc 27:471–477
Kwag S-J, Kim JG, Oh S-T, Kang W-K (2013) Single incision vs. conventional laparoscopic anterior resection for sigmoid colon cancer: a case-matched study. Am J Surg 206:320–325
Pedraza R, Aminian A, Nieto J, Faraj K, Pickron TB, Haas EM (2013) Single-incision laparoscopic colectomy for cancer: short-term outcomes and comparative analysis. Minim Invasive Surg. doi:10.1155/2013/283438
Vasilakis V, Clark CE, Liasis L, Papaconstantinou HT (2013) Noncosmetic benefits of single-incision laparoscopic sigmoid colectomy for diverticular disease: a case-matched comparison with multiport laparoscopic technique. J Surg Res 180:201e–207e
Yun J-A, Yun SH, Park YA, Cho YB, Kim HC, Lee WY, Chun HK (2013) Single-incision laparoscopic right colectomy compared with conventional laparoscopy for malignancy: assessment of perioperative and short-term oncologic outcomes. Surg Endosc 27:2122–2130
Currò G, Cogliandolo A, Lazzara S, La Malfa G, Navarra G (2012) Single-incision versus three-port conventional laparoscopic right hemicolectomy: is there any real need to go single? J Laparoendosc Adv Surg Tech 22:621–624
Egi H, Hattori M, Hinoi T, Takakura Y, Kawaguchi Y, Shimomura M, Tokunaga M, Adachi T, Urushihara T, Itamoto T, Ohdan H (2012) Single-port laparoscopic colectomy versus conventional laparoscopic colectomy for colon cancer: a comparison of surgical results. World J Surg Oncol 10:61. doi:10.1186/1477-7819-10-61
Fujii S, Watanabe K, Ota M, Watanabe J, Ichikawa Y, Yamagishi S, Tatsumi K, Suwa H, Kunisaki C, Taguri M, Morita S, Endo I (2012) Single-incision laparoscopic surgery using colon-lifting technique for colorectal cancer: a matched case–control comparison with standard multiport laparoscopic surgery in terms of short-term results and access instrument cost. Surg Endosc 26:1403–1411
Gaujoux S, Maggiori L, Bretagnol F, Ferron M, Panis Y (2012) Safety, feasibility, and short-term outcomes of single port access colorectal surgery: a single institutional case-matched study. J Gastrointest Surg 16:629–634
Huscher CG, Mingoli A, Sgarzini G, Mereu A, Binda B, Brachini G, Trombetta S (2012) Standard laparoscopic versus single-incision laparoscopic colectomy for cancer: early results of a randomized prospective study. Am J Surg 204:115–120
Katsuno G, Fukunaga M, Lee Y (2012) Single-incision versus conventional laparoscopic colectomy: a case-matched series of 90 cases. Surg Endosc 26:S249–S430
Osborne AJ, Lim J, Gash KJ, Chaudhary B, Dixon AR (2012) Comparison of single-incision laparoscopic high anterior resection with standard laparoscopic high anterior resection. Colorectal Dis 15:329–333
Poon JTC, Cheung CW, Fan JKM, Lo OS, Law WL (2012) Single-incision versus conventional laparoscopic colectomy for colonic neoplasm: a randomized, controlled trial. Surg Endosc 26:2729–2734
Ramos-Valadez DI, Ragupathi M, Nieto J, Patel CB, Miller S, Pickron TB, Haas EM (2012) Single-incision versus conventional laparoscopic sigmoid colectomy: a case-matched series. Surg Endosc 26:2729–2734
Velthuis S, van den Boezem PB, Lips DJ, Prins HA, Cuesta MA, Sietses C (2012) Comparison of short-term surgical outcomes after single-incision laparoscopic versus multiport laparoscopic right colectomy: a two-center, prospective case-controlled study of 100 patients. Dig Surg 29:477–483
Champagne BJ, Lee EC, Leblanc F, Stein SL, Delaney CP (2011) Single-incision vs. straight laparoscopic segmental colectomy: a case-controlled study. Dis Colon Rectum 54:183–186
Chen WT-L, Chang S-C, Chiang H-C, Lo WY, Jeng LB, Wu C, Ke TW (2011) Single-incision laparoscopic versus conventional laparoscopic right hemicolectomy: a comparison of short-term surgical results. Surg Endosc 25:1887–1892
Kim S-J, Ryu GO, Choi B-J, Kim JG, Lee KJ, Lee SC, Oh ST (2011) The short-term outcomes of conventional and single-port laparoscopic surgery for colorectal cancer. Ann Surg 254:933–940
Lai CW, Edwards TJ, Clements DM, Coleman MG (2011) Single port laparoscopic right colonic resection using a ‘vessel-first’ approach. Colorectal Dis 14:1138–1144
Lee SW, Milsom JW, Nash GM (2011) Single-incision versus multiport laparoscopic right and hand-assisted left colectomy: a case-matched comparison. Dis Colon Rectum 54:1355–1361
Lu C-C, Lin SE, Chung K-C, Rau K-M (2011) Comparison of clinical outcome of single-incision laparoscopic surgery using a simplified access system with conventional laparoscopic surgery for malignant colorectal disease. Colorectal Dis 14:e171–e176
McNally ME, Moore BT, Brown KM (2011) Single-incision laparoscopic colectomy for malignant disease. Surg Endosc 25:3559–3565
Papaconstantinou HT, Scott TJ (2011) Single-incision laparoscopic colectomy for cancer: assessment of oncologic resection and short-term outcomes in a case-matched comparison with standard laparoscopy. Surgery 150:820–827
Wolthuis AM, Penninckx F, Fieuws S, D’Hoore A (2011) Outcomes for case-matched single-port colectomy are comparable with conventional laparoscopic colectomy. Colorectal Dis 14:634–641
Adair J, Gromski MA, Lim RB, Nagle D (2010) Single-incision laparoscopic right colectomy: experience with 17 consecutive cases and comparison with multiport laparoscopic right colectomy. Dis Colon Rectum 53:1549–1554
Gandhi DP, Ragupathi M, Patel CB, Ramos-Valadez DI, Pickron TB, Haas EM (2010) Single-incision versus hand-assisted laparoscopic colectomy: a case-matched series. J Gastrointest Surg 14:1875–1880
Waters JA, Guzman MJ, Fajardo AD, Selzer DJ, Wiebke EA, Robb BW, George VV (2010) Single-port laparoscopic right hemicolectomy: a safe alternative to conventional laparoscopy. Dis Colon Rectum 53:1467–1472
Keshava A, Young CJ, Richardson GL, De-Loyde K (2013) A historical comparison of single incision and conventional multiport laparoscopic right hemicolectomy. Colorectal Dis 15:e618–e622
Lim SW, Kim HR, Kim YJ (2014) Single incision laparoscopic colectomy for colorectal cancer: comparison with conventional laparoscopic colectomy. Ann Surg Treat Res 87:131–138
Khayat A, Maggiori L, Vicaut E, Ferron M, Panis Y (2015) Does single port improve results of laparoscopic colorectal surgery? A propensity score adjustment analysis. Surg Endosc. doi:10.1007/s00464-015-4063-7
Yang TX, Chua TC (2012) Single-incision laparoscopic colectomy versus conventional multiport laparoscopic colectomy: a meta-analysis of comparative studies. Int J Colorectal Dis 28:89–101
Maggiori L, Gaujoux S, Tribillon E, Bretagnol F, Panis Y (2012) Single-incision laparoscopy for colorectal resection: a systematic review and meta-analysis of more than thousand procedures. Colorectal Dis 14:e643–e654
Li P, Wang DR, Wang LH, Li YK, Chen J (2012) Single-incision laparoscopic surgery vs. multiport laparoscopic surgery for colectomy: a meta-analysis of eleven recent studies. Hepatogastroenterology 59:1345–1349
Lujàn JA, Soriano MT, Abrisqueta J, Pérez D, Parrilla P (2015) Single-port colectomy vs. multi-port laparoscopic colectomy. Systematic review and meta-analysis of more than 2800 procedures. Cir Esp 93:307–319
Hassan I, You YN, Cima RR, Larson DW, Dozois EJ, Barnes SA, Pemberton JH (2008) Hand-assisted versus laparoscopic-assisted colorectal surgery: practice patterns and clinical outcomes in a minimally invasive colorectal practice. Surg Endosc 22:739–743
Marcello PW, Fleshman JW, Milsom JW, Read TE, Arnell TD, Birnbaum EH, Feingold DL, Lee SW, Mutch MG, Sonoda T, Yan Y, Whelan RL (2008) Hand-assisted laparoscopic vs. laparoscopic colorectal surgery: a multicenter, prospective, randomized trial. Dis Colon Rectum 51:818–826
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008
Pisanu A, Porceddu G, Reccia I, Saba A, Uccheddu A (2013) Meta-analysis of studies comparing single-incision laparoscopic appendectomy and conventional multiport laparoscopic appendectomy. J Surg Res 183:e49–e59
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Champagne BJ, Papaconstantinou HT, Parmar SS, Nagle DA, Young-Fadok TM, Lee EC, Delaney CP (2012) Single-incision versus standard multiport laparoscopic colectomy. A multicenter, case-controlled comparison. Ann Surg 255:66–69
Papaconstantinou HT, Sharp N, Thomas JS (2011) Single-incision laparoscopic right colectomy: a case-matched comparison with standard laparoscopic and hand-assisted laparoscopic techniques. J Am Coll Surg 213:72–82
Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Makino T, Milsom JW, Lee SW (2012) Feasibility and safety of single-incision laparoscopic colectomy. Ann Surg 255:667–676
Leblanc F, Champagne BJ, Augestad KM, Stein SL, Marderstein E, Reynolds HL, Delaney CP (2010) Single incision laparoscopic colectomy: technical aspects, feasibility, and expected benefits. Diagn Ther Endosc. doi:10.1155/2010/913216
Keller DS, Ibarra S, Flores-Gonzalez JR, Ponte OM, Madhoun N, Pickron TB, Haas EM (2015) Outcomes for single-incision laparoscopic colectomy surgery in obese patients: a case-matched study. Surg Endosc. doi:10.1007/s00464-015-4268-9
Kim HO, Yoo CH, Lee SR, Son BH, Park YL, Shin JH, Kim H, Han WK (2012) Pain after laparoscopic appendectomy: a comparison of transumbilical single-port and conventional laparoscopic surgery. J Korean Surg Soc 82:172–178
Acknowledgments
We thank Ms. Jessica Thompson, BMsC (University of Dundee, UK) for revising the English.
Author contributions
Podda M was involved in study design, acquisition, interpretation, and analysis of data; drafting and critically revising the article for important intellectual content; and final approval of the version to be published; Saba A was involved in acquisition, interpretation, and analysis of data; drafting and critically revising the article for important intellectual content; and final approval of the version to be published; Porru F was involved in interpretation and analysis of data; drafting and critically revising the article for important intellectual content; and final approval of the version to be published; Pisanu A was involved in study design, acquisition, interpretation, and analysis of data; drafting and critically revising the article for important intellectual content; and final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Mauro Podda, Alessandra Saba, Federica Porru, and Adolfo Pisanu have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Podda, M., Saba, A., Porru, F. et al. Systematic review with meta-analysis of studies comparing single-incision laparoscopic colectomy and multiport laparoscopic colectomy. Surg Endosc 30, 4697–4720 (2016). https://doi.org/10.1007/s00464-016-4812-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4812-2