Abstract
Background
A minimally invasive method of entero-enteral bypass may be desirable for treatment of obstruction, obesity, or metabolic syndrome. We have developed a technology based on miniature self-assembling magnets which create large-caliber anastomoses (incisionless anastomosis system or IAS). The aim of this study was to assess (a) procedural characteristics of IAS deployment and (b) long-term integrity and patency of the resulting jejuno-ileal dual-path bypass.
Methods
Endoscopic jejuno-ileal bypass creation using IAS magnets was performed in 8 Yorkshire pigs survived 3 months. Procedure: The jejunal magnet was endoscopically deployed. However, the ileal magnet required surgical delivery given restraints of porcine anatomy. A 5-mm enterotomy was created through which the ileal magnet was inserted using a modified laparoscopic delivery tool. Magnets were manually coupled. Pigs underwent serial endoscopies for anastomosis assessment. Three-month necropsies were performed, followed by pressure testing of anastomoses and histological analysis.
Results
Jejuno-ileal bypass creation using self-assembling IAS magnets was successful in all 8 pigs (100 %). Patent, leak-free bypasses formed in all animals by day 10. All IAS magnets were expelled by day 12. Anastomoses were widely patent at 3 months, with mean maximal diameter of 30 mm. At necropsy, adhesions were minimal. Pressure testing confirmed superior integrity of anastomotic tissue. Histology showed full epithelialization across the anastomosis with no evidence of submucosal fibrosis or inflammation.
Conclusions
Entero-enteral bypass using self-assembling IAS magnets is safe and technically feasible in the porcine model. IAS magnets can be rapidly delivered endoscopically or through a modified laparoscopic device. Expulsion of fused magnets avoids retention of prosthetic material. Anastomoses are widely patent and fully re-epithelialized. Three-month pressure testing reveals anastomotic tissue to be as robust as native tissue, while necropsy and histology suggests minimal/absent tissue inflammation. In human anatomy, a fully endoscopic jejuno-ileal bypass using IAS magnets may be feasible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A fully endoscopic method for creation of a durable intestinal bypass may be desirable for treatment of obesity, type II diabetes, and malignant obstructions [1–3]. Currently, intestinal bypasses are surgically created using staples or suture [4] and have been difficult to replicate endoscopically [5–8]. A third strategy based on compression anastomosis has been successfully applied endoscopically in animal studies [9–16], and magnetic compression in particular has also been utilized in a human trial as a minimally invasive treatment of malignant gastric outlet obstruction [17]. However, compression anastomoses have historically suffered from two major disadvantages: (1) They require days to form [18], and (2) transoral delivery of the device has restricted anastomosis size (in the literature, maximum diameter 1.4 cm) which in turn has limited long-term patency [19].
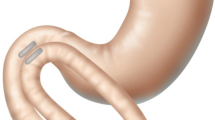
Our group has devised a purely endoscopic device and method for intestinal bypass creation (incisionless anastomosis system or IAS) using compressive force delivered by self-assembling magnets. These “smart” magnets are endoscopically delivered and self-assemble into an octagon capable of creating large-caliber anastomoses (Fig. 1A–D). When reciprocal octagonal magnets occupy adjacent lumens, they couple to form a portal for immediate bypass. Over several days, these magnets fuse, slough off, and are naturally expelled, leaving behind a large side-to-side anastomosis (i.e., dual-pathway anastomosis; Fig. 2A–D).
IAS evolved from technology previously called self-assembling magnets for endoscopy (SAMSEN), which our group used for successful gastrojejunostomy creation in an acute porcine model [20] and durable jejuno-colonic anastomosis in a survival porcine model [21]. The overarching aim of this particular study was to evaluate IAS for entero-enteral anastomosis creation in a long-term survival model. The specific aims of this study were (1) to evaluate the procedural characteristics of the refined IAS design; (2) time required for bypass to form; (3) short- and long-term safety; (4) 3-month patency; (5) integrity of the anastomosis as measured by burst pressure studies; and (6) histological characteristics of the anastomosis.
Methods and materials
This preclinical study focused on defining the procedural characteristics of IAS delivery and the characteristics of the resulting intestinal bypass. IAS technology was developed with the support of the Center for Integration of Medicine and Innovative Technologies (CIMIT; Boston, MA). IAS technology is now owned by GI Windows (W. Bridgewater, MA).
Incisionless anastomosis system
The incisionless anastomosis system (IAS) includes a pair of self-assembling magnetic octagons, each approximating the diameter of small bowel lumen. IAS components were constructed using neodymium–iron–boron rare earth magnets coated in a proprietary bio-safe material. IAS was designed for delivery through a colonoscope (Olympus CF-160AL; Olympus America Inc., Center Valley, PA; instrument channel 3.7 mm diameter).
Survival animal studies
This study was conducted with approval by the Institutional Animal Use and Care Committee at Pine Acres Research Facility (Norton, MA). Survival studies were performed in a total of eight juvenile Yorkshire pigs (30–40 kg).
Pigs were nil per os (NPO) 24 h preprocedure. Animals were initially sedated using intramuscular injection of Telazol, Xylazine, and Atropine. General anesthesia was used.
Enteroscopy was performed for IAS magnet deployment in the proximal jejunum, approximately 30–50 cm beyond the pylorus (Fig. 3). Anatomic constraints (i.e., corkscrew colon in pigs) precluded endoscopic delivery of the ileal IAS magnet; therefore, laparotomy was performed. A 5-mm ileotomy was created between two stay sutures using an electrocautery knife 100 cm proximal to the ileocecal valve. The ileal IAS magnet was then delivered through the ileotomy using a modified laparoscopic delivery device. The ileal IAS magnet was centered around the ileotomy and manually coupled with the jejunal IAS magnet.
All animals were recovered and underwent daily inspection to assess food intake and general appearance. All animals were fed a standard diet throughout. Animals were scoped every 3–5 days to evaluate the intestinal bypass which sometimes involved endoluminal instillation of Omnipaque contrast to perform a double-contrast study for evaluation of anastomotic leak. Fluoroscopy was also used to confirm expulsion of magnets.
In order to confirm reversibility of bypass, the final three pigs underwent surgical resection of their anastomoses and re-establishment of intestinal continuity at 3 months. First, the jejuno-ileal anastomosis was removed by incising the small intestine longitudinally with an electrocautery knife. Then, two stay sutures were placed at the margin of defect, in the middle of incision line. Finally, transverse closure was performed on the defects by layer-to-layer running suture with 4–0 absorbable suture. These animals were survived for another 2 weeks. Laparoscopic reversal with linear stapler was considered, but it was deemed more important to resect the anastomosis en bloc for subsequent burst pressure studies and histological analysis.
Final endoscopic evaluation and necropsies were performed at 3 months in the first five pigs, and 3.5 months for the final 3 pigs. The peritoneal cavity and the anastomosis were grossly inspected for the presence or absence of adhesions. Adhesions were scored on a scale of 0 to 3 according to a previously published scoring system applied to a similar porcine survival model [22]. A score of 0 represented no adhesions, one represented filmy avascular adhesions, two represented dense or vascular adhesions, and three represented dense and vascular adhesions. The anastomosis was then resected en bloc, and the diameter of the anastomosis was recorded. Subsequently, the anastomosis was subjected to underwater air pressure testing using previously published methodology [23]. Sites of tissue failure were recorded. Finally, the tissue was fixed in formalin for histological evaluation.
Results
Survival animal studies
All eight pigs underwent successful IAS magnet deployment, magnet self-assembly, and coupling of reciprocal IAS magnets (Fig. 4). All pigs recovered without incident.
By day 10 in all animals, central tissue necrosis had created a patent bypass within the coupled magnets (Fig. 5A). The anastomosis was robust enough to allow for deep scope passage, and double-contrast studies demonstrated the absence of anastomotic leak in all animals.
By day 90 in all animals, the fully fused IAS magnets had sloughed off and had been naturally expelled, leaving behind a fully patent anastomosis. The anastomosis rim appeared to be completely re-epithelialized (Fig. 5B). Deep endoscopic exploration again showed a widely patent dual-path bypass.
The three pigs that underwent surgical reversal at day 90 recovered without incident. They were able to eat a liquid diet the next day, an ad lib diet at postoperative day 3, and resumed their preoperative growth rates.
Postmortem analysis
All eight pigs exhibited adhesions involving the laparotomy incision, but minimal to absent adhesions at the anastomosis. All adhesions at the anastomosis were scored zero (absent) or one (filmy avascular adhesions) (Fig. 6). Additionally, there was no evidence of hemorrhage or abscess. The mean diameter of the anastomosis was 30 mm (27–35 mm). Upon pressure testing, the site of tissue failure was native tissue and not anastomotic tissue in all eight specimens (Fig. 7). Gross examination of the anastomoses showed intact mucosal surfaces with no evidence of ulceration, fissures, strictures, or abscess formation. Histological analysis demonstrated virtual absence of active inflammation as well as minimal fibrosis and scar formation, all consistent with a well-healed anastomotic site (Fig. 8).
Discussion
The IAS was designed with an eye toward retaining the advantages of compression anastomosis (i.e., lower bleeding and leak rates, lower inflammation and stricture rates, higher mechanical strength) while mitigating the major historical limitations of (1) prolonged time for formation and (2) short-term patency. First, to address the time limitation, IAS magnets were designed to self-assemble into reciprocal pairs of octagonal frames which, if desired, could serve as a portal for immediate endoscopic access. Alternatively, after a few days, the coupled magnets would slough off into the enteral stream and leave behind a robust anastomosis. Second, to address short-term patency, which is a function of magnet size limitations inherent to per-oral delivery (cricopharyngeus), the IAS magnets were designed to self-assemble into much larger macro-configurations that could yield anastomotic calibers approaching the diameter of the small bowel itself, if not larger.
We had previously demonstrated proof-of-concept of self-assembling magnets in an acute animal study for immediate gastrojejunostomy creation [21] as well as a survival animal study for jejuno-colonic anastomosis [22]. In the current animal survival study, we proved that a durable entero-enteral anastomosis could be created using IAS magnets. Furthermore, we proved that the resulting anastomoses were characterized by the following critical features: (1) absence of bleeding, (2) absence of leaks, (3) long-term patency, (4) equivalent if not superior strength compared to native tissue, and (5) the absence of residual foreign material to cause fibrosis or inflammation.
Given the use of magnets, certain precautions need to be followed. First, patients will not be able to undergo magnetic resonance imaging (MRI) studies until expulsion of IAS magnets is confirmed. Second, the use of endoscopic instruments with stainless steel or other ferromagnetic composition will require care around the magnets to avoid inadvertent capture. Notably, standard endoscopes appear to function normally in the presence of IAS magnets.
The main limitations of this present work include small numbers and animal subjects. Additionally, this protocol required surgical assist for ileal magnet deployment given the constraints of porcine anatomy. Nevertheless, we believe the ileal magnet can be delivered endoscopically in human anatomy, but this will have to be born out in a clinical study.
In summary, this study presents successful creation of large-caliber, durable entero-enteral anastomoses using self-assembling IAS magnets in a porcine survival model. While this study utilized a combined endoscopic/surgical strategy for IAS magnet delivery and coupling, a fully endoscopic approach may be feasible in humans.
References
Ly J, O’Grady G, Mittal A, Plank L, Windsor JA (2010) A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 24:290–297
Gentileschi P, Kini S, Catarci M, Gagner M (2002) Evidence-based medicine: open and laparoscopic bariatric surgery. Surg Endosc 16:736–744
Duan J, Tan C, Xu H et al (2015) Side-to-side jejunoileal bypass induces better glucose-lowering effect than end-to-side jejunoileal bypass on nonobese diabetic rats. Obes Surg 25:1458–1467
Himpens JM (2004) The gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass. Semin Laparosc Surg 11:171–177
Kantsevoy SV, Jagannath SB, Niiyama H et al (2005) Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc 62:287–292
Bergstrom M, Ikeda K, Swain P, Park P (2006) Transgastric anastomosis by using flexible endoscopy in a porcine model (with video). Gastrointest Endosc 63:307–312
Chiu PW, Wai Ng EK, Teoh AY et al (2010) Transgastric endoluminal gastrojejunostomy: technical development from bench to animal study (with video). Gastrointest Endosc 71:390–393
Shang E, Hasenberg T, Magdeburg R, Keese M, Post S, Weiner R (2009) First experiences with a circular stapled gastrojejunostomy by a new transorally introducible stapler system in laparoscopic Roux-en-Y gastric bypass. Obes Surg 19:230–236
Swain CP, Mills TN (1991) Anastomosis at flexible endoscopy: an experimental study of compression button gastrojejunostomy. Gastrointest Endosc 37:626–631
Villaverde A, Cope C, Chopita N et al (2004) Long term follow-up of endoscopic gastroenteric anastomoses with magnets (EGAM) [abstract]. Gastrointest Endosc 59:AB92
Fritscher-Ravens A, Mosse CA, Mukherjee D et al (2003) Transluminal endosurgery: single lumen access anastomotic device for flexible endoscopy. Gastrointest Endosc 58:585–591
Jamshidi R, Stephenson JT, Clay JG et al (2009) Magnamosis: magnetic compression anastomosis with comparison to suture and staple techniques. J Pediatr Surg 44:222–228
Pichakron KO, Jelin EB, Hirose S et al (2011) Magnamosis II: magnetic compression anastomosis for minimally invasive gastrojejunostomy and jejunojejunostomy. J Am Coll Surg 212:42–49
Gonzales KD, Douglas G, Pichakron KO et al (2012) Magnamosis III: delivery of a magnetic compression anastomosis device using minimally invasive endoscopic techniques. Pediatr Surg 47:1291–1295
Wall J, Diana M, Leroy J et al (2013) Magnamosis IV: magnetic compression anastomosis for minimally invasive colorectal surgery. Endoscopy 45:643–648
Diana M, Wall J, Perretta S et al (2011) Totally endoscopic magnetic enteral bypass by external guided rendezvous technique. Surg Innov 18:317–320
Chopita N, Vaillaverde A, Cope C et al (2005) Endoscopic gastroenteric anastomosis using magnets. Endoscopy 37:313–317
Cope C (1995) Creation of compression gastroenterostomy by means of the oral, percutaneous, or surgical introduction of magnets: feasibility study in swine. J Vasc Interv Radiol 6:539–545
Cope C, Ginsberg GG (2001) Long-term patency of experimental magnetic compression gastroenteric anastomoses achieved with covered stents. Gastrointest Endosc 53:780–784
Ryou M, Cantillon-Murphy P, Azagury D et al (2011) Smart self-assembling magnets for endoscopy (SAMSEN) for transoral endoscopic creation of immediate gastrojejunostomy (with video). Gastrointest Endosc 73:353–359
Ryou M, Agoston AT, Thompson CC (2015) Endoscopic intestinal bypass creation by using self-assembling magnets in a porcine model. Gastrointest Endosc. doi:10.1016/j.gie.2015.10.023
Romagnuolo J, Morris J, Palesch S et al (2010) Natural orifice transluminal endoscopic surgery versus laparoscopic surgery for inadvertent colon injury repair: feasibility, risk of abdominal adhesions, and peritoneal contamination in a porcine survival model. Gastrointest Endosc 71:817–823
Ryou M, Fong DG, Pai RD et al (2008) Transluminal closure for NOTES: an ex vivo study comparing leak pressures of various gastrostomy and colotomy closure modalities. Endoscopy 40:432–436
Acknowledgments
The incisionless anastomosis system was developed with the support of the Center for Integration of Medicine and Innovative Technologies (CIMIT; Boston, MA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Marvin Ryou, MD: equity interest, GI Windows. Hiroyuki Aihara, MD, Ph.D.: No conflict of interest. Christopher C. Thompson, MD, MHES: equity interest, GI Windows.
Rights and permissions
About this article
Cite this article
Ryou, M., Aihara, H. & Thompson, C.C. Minimally invasive entero-enteral dual-path bypass using self-assembling magnets. Surg Endosc 30, 4533–4538 (2016). https://doi.org/10.1007/s00464-016-4789-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4789-x