Abstract
Background
With increasing interest in natural orifice surgery, there has been a dramatic evolution of transanal and endoluminal surgical techniques. These techniques began with transanal endoluminal surgical removal of rectal masses and have progressed to transanal radical proctectomy for rectal cancer. The first transanal total mesorectal excision (taTME) was performed in 2009 by Sylla, Rattner, Delgado, and Lacy. The improved visibility and working space associated with the taTME technique is intriguing. This video manuscript outlines the training pathway followed by pioneers in the taTME technique, the process of implementation into clinical practice, and initial case report.
Methods
A double board-certified colorectal surgeon with expertise in rectal cancer, minimally invasive total mesorectal excision, transanal endoscopic surgery (TES), and intersphincteric dissection, underwent taTME training in male cadaver models. Institutional review board (IRB) approval for a phase I clinical trial was achieved. The entire operative team including surgeons, nurses, and operative staff underwent taTME cadaver training the day prior to the first clinical case. The case was proctored by an expert in taTME.
Results
A 66-year-old male with uT3N1M0 rectal cancer located in the posterior distal rectum, underwent taTME with laparoscopic abdominal assistance, hand sewn coloanal anastomosis, and diverting loop ileostomy. The majority of the TME was performed transanally with laparoscopic assistance for exposure, splenic flexure mobilization, and high ligation of the vascular pedicles. Operative time was 359 min. There were no intraoperative complications. Pathology revealed a ypT2N1 moderately differentiated invasive adenocarcinoma, grade I TME, 1 cm circumferential radial margin, and 2/13 positive lymph nodes.
Conclusion
Implementation of taTME into practice can be achieved by surgeons with expertise in minimally invasive TME, TES, pre-clinical taTME training in cadavers, case observation, proctoring, and ongoing mentorship. IRB peer review process and participation in a clinical registry are additional measures that should be employed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
The surgical technique associated with the highest rate of cure and lowest rate of rectal cancer recurrence is called “low anterior resection (LAR) with total mesorectal excision (TME)” [1]. The execution of LAR with TME using a minimally invasive surgical approach (laparoscopic, robotic) is a technically challenging operation with reduced working space, retraction capabilities, and visibility. There has been an exceedingly slow adoption rate of this technique with <10 % minimally invasive surgical procedures being performed for rectal cancer in the USA by 2011 [2]. These challenges led to the trend toward increased interest and utilization of robotic rectal surgery. However, even with robotic visualization and instrumentation, there remain several technical hurdles in the minimally invasive approach to rectal cancer. Most notably, the division of the distal rectum remains technically challenging due to the limitations of the pelvic working space and articulation of modern stapler technology.
The standard approach for both minimally invasive and open laparotomy TME for rectal cancer is performed using incisions in the abdomen to access the rectum located deep in the pelvis. Alternatively, the TME can be performed using the anus, a natural orifice, and portal to access the pelvic dissection. Transanal pelvic access for rectal cancer has many potential advantages compared to the transabdominal surgical approach. (1) A transanal approach utilizes pneumatic pressure to assist with the dissection through the avascular embryologic tissue plane surrounding the rectum. This pneumatic pressure dissection does not occur when using a transabdominal approach to rectal surgery. (2) The retraction of the rectum is technically less difficult from the transanal approach as rectal retraction is a “forward pushing motion” for transanal rectal surgery compared to a “pulling up and out of the pelvis motion” required for transabdominal rectal surgery. (3) Rectal division can be performed without using modern endoscopic staplers with a transanal approach. This allows the surgeon to more precisely select the distal margin transection site and perform the transection in a more precise, linear fashion under direct visualization. (4) the low pelvic anastomosis can be performed using a double circular stapler technique or hand sewn technique, thereby avoiding the multiple staple line and staple cross over lines when creating a low pelvic anastomosis that may be associated with an increased rate of anastomotic leak.
The combined transabdominal–transanal (TATA) approach for the surgical management of low-lying rectal cancers was initially described by Marks et al. [3, 4]. The TATA technique was developed in 1984 by Dr. Gerald Marks at Thomas Jefferson University Hospital as an alternative to abdominal perineal resection with permanent end colostomy in patients with low-lying rectal cancers located in the distal third of the rectum [3]. In 2010, Marks et al. [4] reported their laparoscopic TATA experience over a 10-year period. A total of 79 patients underwent laparoscopic TATA resection for locally advanced low-lying rectal cancer located within 3 cm or less of the anorectal ring. There were no perioperative mortalities. The conversion rate was very low (2.5 %), as was the local recurrence rate (2.5 %). All patients underwent a temporary diverting ileostomy at the time of the laparoscopic TATA procedure. After completion of systemic chemotherapy and interval follow-up, 90 % of the patients were able to undergo ileostomy reversal [4].
With increasing interest in natural orifice surgery, there has been an increased interest in the evolution of transanal natural orifice and endoluminal surgical techniques. These techniques began with transanal endoluminal surgical removal of rectal masses [5–11] and have progressed to transanal endoscopic surgical resection of the rectum without abdominal laparoscopic assistance [12–14]. Investigative activity has escalated in the evaluation of proctectomy via a completely transanal approach [15]. The feasibility and safety of transanal proctectomy and transrectal rectosigmoid resection has been demonstrated in human cadavers and porcine survival models using the rigid transanal endoscopic platform [16–24].
The largest cadaveric series investigating transanal rectosigmoid resection for rectal cancer via natural orifice transluminal endoscopic surgery (NOTES) with TME using a rigid transanal endoscopic platform in 32 cadavers was published by Telem and Sylla et al. in 2012 [25]. The majority of patients were male, mean operative time was 5.1 h, and mean specimen length was 53 cm. Transanal dissection alone using the TEM platform was performed in 19 cases (17 cases with the use of a gastroscope). Intra-abdominal assistance was performed using multi-port laparoscopy in eight cases and transgastric endoscopic assistance in five cases. The mesorectum was intact in all of the specimens [25].
The first clinical case utilizing a rigid transanal endoscopic platform to perform a transanal total mesorectal excision (taTME) with laparoscopic assistance in a 76-year-old woman with uT2N2M0 stage III rectal cancer was safely executed by Sylla, Rattner, Delgado, and Lacy in 2009 and published in 2010 [26]. The outcome of this case demonstrated patient safety, accelerated recovery, and equivalent short-term oncologic outcomes. At nearly 4 years of survivorship screening and surveillance, the patient has demonstrated no evidence of locally recurrent or metastatic disease.
However, there are limitations to rigid transanal endoscopic platforms like the transanal endoscopic microsurgery (TEM; Richard Wolf Medical Instruments Corporation, Vernon Hills, IL, USA) and transanal endoscopic operation (TEO; Karl Storz GmbH & Co, Tuttlingen, Germany) platforms including large platform size, rigidity, and prolonged setup time. Atallah et al. [27] demonstrated the innovative use of a single incision laparoscopic (SIL™) port for transanal access in 2010 as an alternative to rigid transanal endoscopic platforms. A new term, transanal minimally invasive surgery (TAMIS) [27], was coined by the group, and the technique has gained widespread interest in the field of colorectal surgery at the national and international level. Many of the disadvantages of the rigid transanal endoscopic platforms have been overcome by the development of soft, disposable transanal endoscopic platforms with the largest taTME experience and series published to date by Antonio Lacy, Barcelona, Spain [27–36].
Training pathway
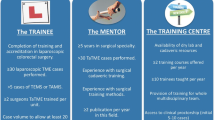
There are six key elements that should be acknowledged and emphasized in the process of taTME training and implementation of the technique into surgical practice: (1) expertise in TME for rectal cancer in your surgical practice [37, 38], (2) expertise in minimally invasive (laparoscopic and/or robotic) TME from the abdominal approach, (3) expertise in transanal endoscopic surgery, (4) experience in intersphincteric dissection for very low rectal invasive neoplasms, (5) practice in taTME techniques in human cadaver models, and (6) institutional review board (IRB) approved data collection with publication of outcomes and/or participation in a clinical registry.
After a few years of experience with laparoscopic and robotic TME for rectal cancer including intersphincteric dissection, the first author trained in TEM and TAMIS [31–34]. Thereafter, following the pathway of pioneers in the taTME technique, the first author and surgical team underwent cadaver training in taTME in 2012. A grade I TME dissection quality was achieved in all eight male cadaver models, of which the results of the first five were published in 2013 [35].
Following cadaver training, a prospective, phase I, clinical trial was then submitted to the IRB and approved. The inclusion criteria were T1–3 N0–2 rectal cancer determined by clinical staging computed tomography (CT) scans and transrectal ultrasound (TRUS), or rectal cancer protocol pelvic MRI. Distal edge of tumor is located within the mid-to-distal rectum (distal edge of tumor no higher than 8–10 cm from the anal verge). Additional inclusion criteria in patients with T3N0 or T1–2N1–2 clinical stage rectal cancer included completion of pre-operative combined 5-FU-based chemotherapy and radiation therapy prior to surgery. Patients with T4, metastatic disease, or primarily anterior lesions were not eligible for this phase I clinical trial. Patients with sphincter involvement on clinical staging TRUS or pelvic MRI were also not eligible.
The day prior to the first scheduled taTME clinical case, a taTME cadaver training day was scheduled to introduce the operating room staff to the steps of the taTME technique. The entire operating room team including surgeons, residents, and nursing staff was present for the taTME cadaver training in which the procedure was performed from incision to closure as it would be in the operative theater (Video 1). A grade I TME dissection was achieved (Fig. 1). An expert in taTME dissection technique proctored the first clinical taTME case at the University of California, San Diego Medical Center in 2013.
Case report
A 66-year-old male with a body mass index (BMI) of 32 kg/m2 was referred for evaluation of rectal bleeding and hemorrhoids and discovered to have a posterior suspicious mass just above the sphincter muscle complex on digital rectal examination. A colonoscopy was performed and confirmed the presence of an invasive adenocarcinoma. Clinical staging was completed and revealed a stage III (uT3N1M0) rectal cancer located in the posterior distal rectum, 2 cm from the anal verge, and occupying approximately 30 % of the circumference of the rectum. The patient underwent combined radiation chemotherapy (4500 cGy with 540 cGy boost; concurrent 5-FU-based radio-sensitizing chemotherapy).
Approximately 10 weeks after completing combined radiation chemotherapy, the patient underwent taTME with laparoscopic abdominal assistance, hand sewn coloanal anastomosis, and diverting loop ileostomy using a two-team approach in August of 2013. The splenic flexure mobilization and high ligation of the inferior mesenteric vein and artery were performed laparoscopically. The TME was performed primarily via a transanal approach with laparoscopic visualization and assistance (Video 2). The operative time was 5 h 59 min (359 min). There were no intraoperative complications. The final pathology revealed ypT2N1 moderately differentiated invasive adenocarcinoma, negative lymphovascular invasion, grade I TME (Fig. 1), 1 cm circumferential radial margin, 0.3 cm distal margin, MSI stable, and 2/13 lymph nodes positive for disease.
The patient’s post-operative course was complicated by high output ileostomy and abdominal abscess which was located at the pelvic brim requiring percutaneous drainage and antibiotic therapy. The abscess may have been due to pulling the completed proctectomy out of the pelvic dissection field and up into the abdominal cavity to inspect the quality of the pelvic dissection after taTME. This maneuver is no longer performed, and there have been no subsequent post-operative abdominal abscess formation. The Foley catheter was removed on post-operative day 3 without urinary retention, and the hospital length of stay was 7 days. The patient completed eight cycles of mFOLFOX6 systemic chemotherapy and has undergone ileostomy reversal. At this time, just over 2 years, the patient does not have any evidence of local recurrence or distant metastasis.
The clinical trial was closed earlier than anticipated due to the recruitment of the primary investigator to another healthcare system. A total of three taTME cases were completed prior to closure of the clinical trial. All three cases were completed without intraoperative complication, grade I TME dissection quality, negative distal margins, circumferential radial margins >1 cm, and lymph node harvests >12. All patients have subsequently undergone ileostomy reversal. The first author has continued to perform taTME in the new healthcare system using a two-team transanal–transabdominal approach for low-lying rectal cancers (10 cm or less from the anal ring) achieving a grade I TME in all twelve cases to date. The second surgeon is a double board-certified colon and rectal surgeon who also underwent taTME cadaver training in May 2014 at the UC San Diego Center for the Future of Surgery. IRB retrospective data collection approval has been obtained, and the perioperative outcome data and short-term oncologic outcomes in a case series report will be submitted for peer review in the future. The first author plans to foster the training and implementation of the taTME technique into the other medical centers within the Southern California Kaiser Permanente Health System in a prospective phase I observational clinical trial setting using the model outlined in this manuscript.
Discussion
taTME is an attractive alternative to current standard minimally invasive TME techniques as the number of abdominal access ports is reduced, the abdominal extraction incision can be avoided in the majority of cases, and the technical difficulty of performing the TME is reduced. Antonio Lacy has the largest experience with taTME for rectal cancer and has published the outcomes of the first 140 taTME cases for rectal cancer [35]. Compared to his laparoscopic experience, the taTME technique is associated with a lower conversion rate (0 vs. 20 %) and shorter TME mean operative times (154 vs. 179 min) [36]. The rate of ileus (4.1, 1.3 %), anastomotic leakage (8.2, 7.3 %), pelvic fluid collection (4.1, 1.3 %), and urinary retention (1.8, 2.7 %) were similar after taTME and laparoscopic TME, respectively.
The order of the surgical steps is widely variable in the literature. The first author prefers to complete the high ligation of the inferior mesenteric vein and artery, splenic flexure mobilization, and proximal mesenteric division laparoscopically followed by a two-team combined transabdominal–transanal TME approach with specimen extraction via the anus when feasible, low pelvic anastomosis, and diverting loop ileostomy when indicated. This combined transanal–transabdominal TME dissection approach has resulted in similar findings of shorter operative times with early initial experience, with the shortest operative time being 295 min thus far (4 h, 55 min; 10–20 taTME case learning phase; unpublished data).
The implementation of this new technique into clinical practice can safely and effectively be achieved by surgeons with expertise in the management of rectal cancer, minimally invasive TME, and transanal endoscopic surgery. Surgeons interested in learning this new technique should have experience with rectal cancer in their surgical practice, expertise in achieving negative circumferential radial margins and grade I TME specimens after a minimally invasive approach to TME, and mastery of transanal endoscopic surgery. Given the relatively recent introduction of the taTME technique, the paucity of long-term oncologic and functional outcomes, and the lack of prospective comparative trials, additional key elements that should be acknowledged and emphasized in the process of taTME training and implementation include pre-clinical taTME cadaver training, case observation, proctoring, mentorship with ongoing feedback, participation in a clinical registry [39], ongoing peer review, and publication of the outcomes after taTME.
References
Heald RJ (1988) The ‘Holy Plane’ of rectal surgery. J R Soc Med 88:503–508
Rea JD, Cone MM, Diggs BS, Deveney KE, Lu KC, Herzig DO (2011) Utilization of laparoscopic colectomy in the United States before and after the clinical outcomes of surgical therapy study group trial. Ann Surg 254:281–288
Marks GJ, Marks JH, Mohiuddin M, Brady L (1998) Radical sphincter-preserving surgery with coloanal anastomosis following high-dose external irradiation for the very low lying rectal cancer. Recent Results Cancer Res 146:161–174
Marks J, Mizrahi B, Dalane S, Nweze I, Marks G (2010) Laparoscopic transanal abdominal transanal resection with sphincter preservation for rectal cancer in the distal 3 cm of the rectum after neoadjuvant therapy. Surg Endosc 24:2700–2707
Buess G, Theiss R, Hutterer F, Pichlmaier H, Pelz P, Holfeld T et al (1983) Die transanale endoscopische Rektumoperation. Erprobung einer neuen Methode im Tierversuch. Leber Magen Darm 13:73
Buess G (1993) Review: transanal endoscopic microsurgery (TEM). J R Coll Surg Edinb 38:239–245
Middleton PF, Sutherland LM, Maddern GJ (2005) Transanal endoscopic microsurgery: a systematic review. Dis Colon Rectum 48:270–284
Moore JS, Cataldo PA, Osler T, Hyman NH (2008) Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum 51:1026–1030
Barendse RM, van den Broek FJ, Dekker E, Bemelman WA, de Graaf EJ, Fockens P, Reitsma JB (2011) Systematic review of endoscopic mucosal resection versus transanal endoscopic microsurgery for large rectal adenomas. Endoscopy 43(11):941–949
Allaix ME, Arezzo A, Caldart M, Festa F, Morino M (2009) Transanal endoscopic microsurgery for rectal neoplasms: experience of 300 consecutive cases. Dis Colon Rectum 52:1831–1836
Guerrieri M, Baldarelli M, de Sanctis A, Campagnacci R, Rimini M, Lezoche E (2010) Treatment of rectal adenomas by transanal endoscopic microsurgery: 15 years’ experience. Surg Endosc 24:445–449
Lacy AM, Adelsdorfer C (2011) Totally transrectal endoscopic total mesorectal excision (TME). Colorectal Dis 13(Suppl. 7):43–46
Gaujoux S, Bretagnol F, Au J, Ferron M, Panis Y (2011) Single port access proctectomy with total mesorectal excision and intersphincteric resection with a primary transanal approach. Colorectal Dis 13(9):e305–e307
Zhang H, Zhang YS, Jin XW, Li MZ, Fan JS, Yang ZH (2013) Transanal single-port laparoscopic total mesorectal excision in the treatment of rectal cancer. Tech Coloproctol 17(1):117–123
Sylla P (2010) Current experience and future directions of completely NOTES colorectal resection. World J Gastrointest Surg 2(6):193–198
Whiteford MH, Denk PM, Swanstrom LL (2007) Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc 21(10):1870–1874
Denk PM, Swanstrom LL, Whiteford MH (2008) Transanal endoscopic microsurgical platform for natural orifice surgery. Gastrointest Endosc 68(5):954–959
Fajardo AD, Hunt SR, Fleshman JW, Mutch MG (2010) Transanal single-port low anterior resection in a cadaver model. Surg Endosc 24:1765
Trunzo JA, Delaney CP (2010) Natural orifice proctectomy using a transanal endoscopic microsurgical technique in a porcine model. Surg Innov 17(1):48–52
Sylla P, Sohn DK, CizqinerS KonukY, Turner BG, Gee DW, Willingham FF, Hsu M, Mino-Kenudson M, Brugge WR, Rattner DW (2010) Survival study of natural orifice transluminal endoscopic surgery for rectosigmoid resection using transanal endoscopic microsurgery with or without transgastric endoscopic assistance in a swine model. Surg Endosc 24(8):2022–2030
Sylla P, Willingham FF, Sohn DK, Gee D, Brugge WR, Rattner DW (2008) NOTES rectosigmoid resection using transanal endoscopic microsurgery (TEM) with transgastric endoscopic assistance: a pilot study in swine. J Gastrointest Surg 12(10):1717–1723
Han Y, He YG, Zhang HB, Lv KZ, Zhang YJ, Lin MB, Yin L (2013) Total laparoscopic sigmoid and rectal surgery in combination with transanal endoscopic microsurgery: a preliminary evaluation in China. Surg Endosc 27(2):518–524
Bhattacharjee HK, Kirschniak A, Storz P, Wilhelm P, Kunert W (2011) Transanal endoscopic microsurgery-based transanal access for colorectal surgery: experience on human cadavers. J Laparoendosc Adv Surg Tech A 21:835–840
Sohn DK, Jeong SY, Park JW, Kim JS, Hwang JH, Kim DW, Kang SB, Oh JH (2011) Comparative study of NOTES rectosigmoidectomy in a swine model: E-NOTES vs P-NOTES. Endoscopy 43(6):526–532
Sylla P, Rattner DW, Delgado S, Lacy AM (2010) NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 24(5):1205–1210
Telem DA, Han KS, Kim MC, Ajari I, Sohn DK, Woods K, Kapur V, Sbeih MA, Perretta S, Rattner DW, Sylla P (2013) Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc 27(1):74–80
Atallah S, Albert M, Larach S (2010) Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 24(9):220–225
Cid RC, Perez JC, Elosua TG (2011) Transanal resection using a single port trocar: a new approach to NOTES. Cir Esp 89:20–23
Dumont F, Goéré D, Honoré C, Elias D (2012) Transanal endoscopic total mesorectal excision combined with single-port laparoscopy. Dis Colon Rectum 55(9):996–1001
Fajardo AD, Hunt SR, Fleshman JW, Mutch MG (2010) Video. Transanal single-port low anterior resection in a cadaver model. Surg Endosc 24:1765
McLemore EC, Coker A, Jacobsen G, Talamini MA, Horgan S (2013) eTAMIS: endoscopic visualization for transanal minimally invasive surgery. Surg Endosc 27(5):1842–1845
McLemore EC, Coker A, Leland H, Yu PT, Devaraj B, Jacobsen G, Talamini MA, Horgan S, Ramamoorthy S (2013) New disposable transanal endoscopic surgery platform: longer channel, longer reach. Glob J Gastroenterol Hepatol 1:36–40
McLemore EC, Weston LA, Coker AM, Jacobsen GR, Talamini MA, Horgan S, Ramamoorthy SL (2014) Transanal minimally invasive surgery for benign and malignant rectal neoplasia. Am J Surg 208(3):372–381
McLemore EC, Leland H, Devaraj B, Pola S, Docherty MJ, Patel DR, Levesque BG, Sandborn WJ, Talamini MA, Ramamoorthy SL (2013) Transanal endoscopic surgical proctectomy for proctitis case series report: diversion, radiation, ulcerative colitis, and Crohn’s disease. Glob J Gastroenterol Hepatol 1:41–47
McLemore EC, Coker A, Devaraj B, Chakedis J, Maawy A, Inui T, Talamini MA, Horgan S, Peterson MR, Sylla P, Ramamoorthy S (2013) TAMIS assisted laparoscopic low anterior resection with total mesorectal excision in a cadaveric series. Surg Endosc 27:3478–3484
Lacy AM, Tasende MM, Delgado S, Fernandez-Hevia M, Jimenez M, DeLacy B, Castells A, Bravo R, Wexner SD, Heald RJ (2015) Transanal total mesorectal excision for rectal cancer: outcomes after 140 patients. J Am Coll Surg 221(2):415–423
Hohenberger W, Merkel S, Hermanek P (2013) Volume and outcome in rectal cancer surgery: the importance of quality management. Int J Colorectal Dis 28(2):197–206
Wexner SD, Rotholtz NA (2000) Surgeon influenced variables in resectional rectal cancer surgery. Dis Colon Rectum 43(11):1606–1627
Hompes R, Arnold S, Warusavitarne J (2014) Towards the safe introduction of transanal total mesorectal excision: the role of a clinical registry. Colorectal Dis 16(7):498–501
Acknowledgments
The authors wish to acknowledge Steven Wexner, MD, Dana Sands, MD, and Eric Weiss, MD, Cleveland Clinic Florida, for their dedication to training and mentoring colorectal surgery residents as well as their ongoing interest, support, and earnest enthusiasm toward advancing the field of minimally invasive colon and rectal surgery. The authors acknowledge the multitude of simulation and training thought leaders within the field of industry who have supported the taTME cadaver surgical training courses including but not limited to: Richard Wolf Medical Instruments Corporation, Karl Storz GmbH & Co, Applied Medical, Covidien/Medtronic, Nodadaq Inc., Stryker Endoscopy, and Novatract. The authors respectfully acknowledge Antonio Lacy for his excellent surgical technique, scientific approach to the evaluation and implementation of the taTME technique into clinical practice, and ongoing training, proctoring, and mentorship within the rectal cancer surgical community.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. McLemore reports grants from Applied Medical, grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study; personal fees from Applied Medical, personal fees from Novadaq, personal fees from Cubist, personal fees from Genomic Health, personal fees from Ethicon Endosurgery, personal fees from Covidien, outside the submitted work. Dr. Harnsberger has nothing to disclose. Dr. Broderick reports grants from Applied Medical, grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study. Dr. Leland reports grants from Applied Medical, grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study. Dr. Sylla has nothing to disclose. Dr. Coker reports grants from Applied Medical, grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study. Dr. Fuchs reports grants from Applied Medical, grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study. Dr. Jacobsen reports grants from Applied Medical, grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study; personal fees from ValenTx, Inc., personal fees from Gore, personal fees from Davol, personal fees from Ethicon Endosurgery outside the submitted work. Dr. Sandler reports grants from Applied Medical, grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study; personal fees from ValenTx, Inc., personal fees from Gore, personal fees from Davol outside the submitted work. Dr. Attaluri has nothing to disclose. Mrs. Tsay, NP, has nothing to disclose. Dr. Wexner reports other from Covidien, personal fees from Karl Storz Endoscopy America, other from Karl Storz Endoscopy America, during the conduct of the study. Dr. Talamini reports grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study. Dr. Horgan reports grants from Applied Medical, grants from Applied Medical, grants from Covidien, grants from Stryker Endoscopy, grants from Novatract, during the conduct of the study.
Additional information
This video abstract received the 2014 SAGES Foundation Gerald Marks Rectal Cancer Award 4/2/14, SAGES Annual Meeting and Scientific Session, Salt Lake City, UT, USA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Cadaveric taTME Dissection. Transanal total mesorectal excision in a male cadaver model (MP4 89577 kb)
Clinical taTME Dissection. Transanal total mesorectal excision, clinical case report and pelvic dissection (MP4 171823 kb)
Rights and permissions
About this article
Cite this article
McLemore, E.C., Harnsberger, C.R., Broderick, R.C. et al. Transanal total mesorectal excision (taTME) for rectal cancer: a training pathway. Surg Endosc 30, 4130–4135 (2016). https://doi.org/10.1007/s00464-015-4680-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4680-1