Abstract
Background
Treatment response to neoadjuvant therapy is histologically associated with more or less intensive inflammation and fibrosis. In consequence, accuracy of endosonographic TN-tumor staging after neoadjuvant treatment is hampered. We analyzed whether the kind of treatment chosen [chemoradiotherapy (CRT) or chemotherapy (CT)] differently influences the accuracy of endoscopic ultrasound after neoadjuvant therapy in esophageal cancer.
Methods
We performed serial endoscopic ultrasound examinations in 18 patients after neoadjuvant CRT and 30 patients after neoadjuvant CT. TN-stage was classified according to the standard parameter. Histological examination of the surgical resection specimen served as gold standard.
Results
The most frequent error was overstaging, especially in patients with complete tumor response or minimal residual disease. Accuracy of T-staging was significantly worse after CRT (0.16) than after CT (0.43), obviously due to difficulty in distinguishing residual tumor from treatment-associated fibrosis and inflammation. Accuracy of N-staging was also hampered, but to a less extent (sensitivity/specificity 0.85/0.36 after CRT, and 0.5/0.42 after CT).
Conclusions
Accuracy of endosonographic TN-tumor staging is significantly more hampered by neoadjuvant CRT than after CT. However, endoscopic ultrasound is insufficient for TN-staging irrespective of the kind of neoadjuvant therapy performed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endoscopic ultrasound (EUS) is generally considered the best technique for local tumor staging in esophageal carcinoma, with a sensitivity of 85–95 % for T-stage [1]. In particular, differentiation between locally limited and locally extended tumors (T3-4 Nx; or Tx N+) is possible with high accuracy [2, 3]. This differentiation is a cornerstone in the diagnostic process, because the latter group will benefit from neoadjuvant therapy before resection [4].
Endosonographic tumor staging is based on several parameters, like tumor infiltration depth into the different esophageal wall layers, irregularity of outer tumor border, or lymph node size and echogenicity. On the other hand, EUS often cannot reliably differ between neoplastic and inflammatory tissue.
Neoadjuvant therapy often results in a histologically proven tumor regression. After chemoradiotherapy, a complete tumor response can be observed in up to 40 % of cases [5]. Histologically, tumor regression is accompanied by a more or less intensive fibrotic and inflammatory tissue alteration. In addition, inflammatory response due to neoadjuvant therapy may induce local lymphatic reaction, leading to lymphonodular disease. According to this, impaired accuracy of EUS with relevant overstaging after neoadjuvant chemoradiotherapy due to the difficulty in distinguishing residual tumor from radiation fibrosis has been reported [6–11].
In opposite to chemoradiotherapy, histological changes may be less pronounced after chemotherapy alone, as the rate of complete pathohistological tumor regression after chemotherapy is lower [12]. There is less evidence in the literature about endosonographic tumor staging accuracy after neoadjuvant chemotherapy [13, 14], than after chemoradiotherapy, without a direct comparison between these both treatment modalities published up to now. Therefore, we analyzed in our collective whether a relevant difference in the accuracy of uTN staging after neoadjuvant chemotherapy and chemoradiotherapy can be observed.
Materials and methods
This study is a retrospective chart review. We analyzed 48 patients with locally advanced esophageal or esophagogastric junction tumor, who underwent neoadjuvant chemotherapy (CT, 30) or chemoradiotherapy (CRT, 18) before resection (Table 1). The study group consisted of 37 patients with adenocarcinoma, 10 with squamous cell carcinoma, and one patient with a small cell carcinoma. In 33 patients, the tumor was located in the tubular esophagus and in 15 patients at the esophagogastric junction.
The first EUS was performed before initiation of neoadjuvant therapy. The second EUS was done within 2 weeks after completion of neoadjuvant therapy in 40 patients, and after more than 2 weeks time in eight patients (Table 1). The endosonographic uT-stage was analyzed according to the established parameters: uT1, tumor involvement up to the second echorich layer (mucosa and submucosa); uT2, infiltration into the second echopoor layer (m. propria); uT3, extension of the tumor outside the m. propria with irregular outer border; and uT4, infiltration of adjacent organs, like aorta, or tracheo-bronchial system). Locoregional lymph nodes were classified according to the criteria of Catalano et al. [15]: hypoechoic texture, round shape, sharp border, diameter >10 mm. The results of lymph node staging were summarized as N+ (nodal positive) and N− (nodal negative). The prefix “u” indicates the initial endosonographic stage before neoadjuvant treatment, and the prefix “yu” indicates the endosonographic tumor stage after neoadjuvant treatment. EUS-guided fine-needle aspiration of regional lymph nodes was not performed.
EUS was performed using radial scanner (mechanical scanner: MH908, diameter 7.9 mm; UM 160, diameter 12.7 mm; electronical scanner: UE 160, diameter 11.8 mm; all Olympus Germany, Hamburg). Scanning frequencies were 7.5 and 12 MHz. Choice of the echoendoscope was at the discretion of the examiner and especially dependent from the degree of tumor stenosis. In all examinations, tumor stenosis could be passed; therefore, a complete endosonographic examination including all relevant lymphnode sites was possible in all patients. The examinations were performed by four experienced investigators. The Katharinenhospital is a municipal tertiary center with an annual load of approximately 1000 endoscopic ultrasound examinations.
All patients underwent curative resection. Esophagectomy specimens were examined to determine the pT- and pN-stage. The prefix “y” according to the TNM classification indicates that the patient has had neoadjuvant therapy before operation. TN classification was done according to the 6th edition of the UICC TNM staging system. The EUS-determined T- and N-stage was compared with the postsurgical pathologic stage for each patient.
For statistical analysis, sensitivity (true positives/true positives + false negatives), specificity (true negatives/true negatives + false positives), and accuracy (true positives + true negatives/total cases) were calculated.
Results
Initial EUS was performed in 46 patients and revealed the following tumor stages: one uT1, 41 uT3, 4 uT4, 37 uN+, 9 uN−. Apart from more patients having nodal positive disease (N+) in the group treated with radio-chemotherapy, there was no substantial difference between the both groups (Table 1). Endosonography after neoadjuvant therapy showed the following results (CT/CRT): yuT0: 3 (2/1); yuT1: 2 (2/0); yuT2: 15 (9/6); yuT3: 28 (17/11); yuT4: 0. N-Stage (CT/CRT): N−: 19 (14/5); N+: 29 (16/13). Therefore, according to endosonographic TN-staging, tumor regression according to uT-Stage (23 patients) and uN-stage (10 patients) could be observed in approximately half of the patients, without relevant difference between the treatment groups (Fig. 1). Two patients treated with chemotherapy and initially staged as uN0 developed significant lymph nodes after neoadjuvant treatment. However, in one of them, in the postsurgical specimen no nodal metastasis was present.
A Stage shift of endosonographic tumor stage (uTN-stage) before and after neoadjuvant therapy (all patients). B Stage shift of endosonographic tumor stage (uTN-stage) before and after neoadjuvant chemotherapy. C Stage shift of endosonographic tumor stage (uTN-stage) before and after neoadjuvant chemoradiotherapy
Pathohistological examination revealed the following tumor stages (CT/CRT): ypT0: 9 (2/7); ypT1: 8 (5/3); ypT2: 8 (3/5), ypT3: 23 (20/3); ypT4: 0; ypN−: 25 (14/11); ypN+: 23 (16/7). Tumor regression was more pronounced after CRT (ypT0: 7/18) than after CT (ypT0: 2/30). The difference according to N-stage was less pronounced (ypN0 11/18 vs. 14/30) (Fig. 2).
The correlation between yu- and yp-stage is summarized in Tables 2, 3 and 4. In the combined analysis, overall accuracy was poor (T-stage: 0.29; N-stage: 0.5). In the chemotherapy group, the corresponding values were 0.43 and 0.46, respectively. In contrast, in the group treated with chemoradiotherapy, the accuracy of T-stage was substantially worse (0.16), but comparable according to N-stage (0.55).
The most common problem of EUS was overstaging of T-stage (Fig. 3). This occurred more often after CRT (12/18), than after CT (8/30), and especially in case of substantial tumor response, 20/25 ypT0-2 tumors were overstaged, compared to none of the 23 ypT3-tumors. In 11 patients, the tumor was understaged, most often in case of ypT3-tumors. It is remarkable that none of the ypT0-1 tumors, irrespective of the type of neoadjuvant treatment performed, was correctly classified by endosonography.
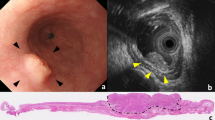
A EUS after neoadjuvant chemoradiotherapy; overstaging (yuT2–ypT0); the prefix yu denotes endosonographic tumor stage after neoadjuvant therapy; yp denotes pathohistological stage after neoadjuvant therapy. B EUS after neoadjuvant chemoradiotherapy; correct staging (yuT3–ypT3); the prefix yu denotes endosonographic tumor stage after neoadjuvant therapy; yp denotes pathohistological stage after neoadjuvant therapy
In N-stage, overstaging occurred in 8/30 patients after chemotherapy and in 7/18 after chemoradiotherapy. Understaging occurred more often after chemotherapy (8/30) than after chemoradiotherapy (1/18). Sensitivity for lymph node metastasis was 0.6 in the entire group, 0.5 after chemotherapy and 0.85 after CRT. The corresponding specificity was 0.4, 0.42 and 0.36.
Discussion
As described before, accuracy of endosonographic tumor staging using the TN classification system is substantially hampered by neoadjuvant therapy and, according to other studies, not comparable to initial tumor staging before neoadjuvant treatment [16, 17]. During the last years, several reports about the accuracy of endosonographic tumor staging in cancer of the esophagus and esophagogastric junction after neoadjuvant chemoradiotherapy have been published with disappointing results [6–11, 16]. Accuracy of endosonographic T-stage ranged between 27 and 47 %, with four studies below the 30 % line. The accuracy in our study is even worse.
Tumor destruction by chemoradiotherapy is histologically accompanied by an inflammatory response and the development of large areas of scarring. Due to this, tumor destruction can result in shrinkage [18], but will often not lead to a restoration of the normal esophageal wall layers. On the other hand, endosonographic tumor staging is based on integrity and disturbance of esophageal wall layers. But endoscopic ultrasound is unable to distinguish viable tumor from necrosis or inflammation. As a result, overstaging is the most relevant problem in staging after neoadjuvant therapy, especially in case of substantial tumor response, as shown in our study by the extreme inaccuracy, especially in low posttherapeutic tumor stages. According to this, most studies reported an overstaging especially in patients with complete response or minimal residual disease [7, 10, 19].
It is mentioned that accuracy of endosonographic T-staging after neoadjuvant therapy depends on the interval between end of treatment and endosonographic examination. Several authors suggest an interval of more than 2 weeks time appropriate, because accuracy would increase due to the resolution of therapy-induced inflammation [13]. In our study, most patients were examined within 2 weeks after completion of neoadjuvant therapy. Therefore, the worse results of our study might be in part explained by this short interval. However, there was no difference between these two groups—more or less than 2 weeks time between end of neoadjuvant therapy and endoscopic ultrasound—according to the accuracy of endosonographic tumor staging. Furthermore, even in studies with a substantial longer interval between neoadjuvant treatment and EUS with up to 6 weeks [8, 11, 16], accuracy of EUS showed no better results. Therefore, expanding the interval between end of treatment and endoscopic ultrasound is not a suitable way to improve the accuracy of endosonographic tumor staging after neoadjuvant treatment.
There are only few reports about the accuracy of endoscopic ultrasound after neoadjuvant chemotherapy alone [13, 14, 16, 20, 21], dealing with patients with cancer of the esophagus or the esophagogastric junction. Accuracy of T-staging ranged between 39 and ~60 %, with the best accuracy (80 %) in the smallest study [21]. Machlenin et al. [21] applied only a doublet chemotherapy (5-FU, cisplatin), and none of their patients developed a therapy response in the second EUS, whereas Mesemas [13] and Misra [14] used chemotherapy triplets, containing cisplatin, 5-FU, and docetaxel or epirubicin, as was the case in our study. These latter chemotherapy regimes are more effective leading to a complete tumor response in up to 15 % of patients [12]. As overstaging after substantial tumor regress is the most relevant problem of staging after neoadjuvant therapy, the excellent result in the study from Machlenkin et al. is obviously due to the fact that only very few of their patients substantially respond to the neoadjuvant therapy.
Without a direct comparative study published before, the literature reveals a slightly better accuracy in T-staging after chemotherapy (range 39–66 %) [13, 14], than after chemoradiotherapy (range 27–47 %) [6–11]. Comparison of different studies with different collectives is always questionable. However, according to our study, our results with direct comparison between the effect of chemotherapy and chemoradiotherapy confirm this assumption: besides the fact that after both therapeutic scenarios, endoscopic ultrasound is not able to identify T-stage with sufficient accuracy, our T-stage-accuracy of 0.43 after neoadjuvant chemotherapy is substantially better than after chemoradiotherapy (0.16). Obviously, this is due to the lesser extent of complete tumor destruction accompanied with less inflammatory and scarring alterations after chemotherapy than after chemoradiotherapy.
Congruent with the results of other studies, endoscopic ultrasound is slightly better in nodal staging after neoadjuvant therapy than in the discrimination of T-stage. Endosonographic criteria of lymph node metastasis are principally questionable. We used the most commonly accepted classification published in 1994 by Catalano et al. (hypoechoic texture, round shape, sharp border, diameter >10 mm; all 4 criteria necessary for nodal positive disease) [15] and did not change the criteria before and after neoadjuvant treatment. Catalano studied patients without neoadjuvant therapy. In their study, accuracy increased up to 100 % with the number of criteria present in a special node, with hypoechogenicity being the most important and size the least useful marker [15]. There are no established endosonographic criteria for metastatic lymph node involvement after neoadjuvant therapy. Most studies used the Catalano criteria [11]; however, to a different degree, in some studies, lymphnode metastasis was suspected even when at least one Catalano criteria was present [6, 16]. In other reports, a diameter of >5 mm was chosen [13, 14, 19, 21]. However, all these studies showed similar results according to N-stage, with an accuracy between 58 and 64 %. Therefore, there is still a need for reliable endosonographic parameters for lymph node metastasis after neoadjuvant treatment besides the possible impact of EUS-FNA [22].
In this study, we used radial scanner of different diameters. Therefore, a complete tumor examination was possible in all patients even in case of cancer stenosis. Furthermore, echoendoscopes with frequency of 7.5 and 12 MHz, which are also used in the vast majority of studies dealing with endosonographic tumor staging, enabled the visualization of all locoregional nodal sites [23]. High-frequency miniprobe ultrasound, with 20 MHz frequency, results in higher-resolution imaging, with better discrimination especially of lower tumor stages. However, high-frequency ultrasound is associated with limited depth of penetration and hampered nodal staging. Contrary to the study of Menzel et al. [24], in our experience, penetration of miniprobes is not sufficient for complete nodal staging. Radial scanners were also used by all other groups except Giovannini [25], who used a linear probe. However, the slightly better results from this group are not directly comparable to the rest of the literature, due to a T-staging system exclusively used by this group. Electronic scanners, as used in 15 of our patients, have a better spacial resolution than the older mechanical probes used in virtually all studies published. However, in our study, there was no correlation between T-stage accuracy and the echoendoscope chosen.
Our study has several limitations: retrospective design, single center study and relatively low case number. These limitations notwithstanding, our data reveal that impaired accuracy of endosonographic tumor staging is more pronounced after neoadjuvant chemoradiotherapy, than after neoadjuvant chemotherapy. Nevertheless, endoscopic ultrasound is insufficient for T- and N-staging after neoadjuvant therapy in esophageal cancer, regardless of the kind of neoadjuvant treatment chosen.
References
Puli SR, Reddy JB, Antillon D, Ibdah JA, Antillon MR (2008) Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 14:1479–1490
Wang KK, Wongkeeson ME, Buttar NS (2005) American gastroenterological association technical review on the role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology 128:1471–1505
Van Vliet EPM, Heijenbrok-Kal MH, Hunink MGM, Kuipers EJ, Siersema PD (2008) Staging investigations for oesophageal cancer: a meta-analysis. B J Cancer 98:547–557
Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D, on behalf of the ESMO Guidelines Working Group (2013) Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24((Supplement 6)):vi51–vi56
Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R (2008) Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26:1086–1092
Agarwal B, Swicher S, Ajani J, Kelly K, Fannin C, Komaki RR, Putnam JB, Abu-Hamda E, Molke KL, Walsch GL, Correa AM, Ho L, Liao Z, Lynch PM, Rice DC, Smythe WR, Stevens CW, Vaporciyan AA, Yao J, Roth JA (2004) Endoscopic ultrasound after preoperative chemoradiation can help identify patients who benefit maximally after surgical esophageal resection. Am J Gastroenterol 99:1258–1266
Willis J, Cooper GS, Isenberg G, Sivak MV, Levitan N, Clayman J, Chak A (2002) Correlation of EUS measurement with pathologic assessment of neoadjuvant therapy in response in esophageal carcinoma. Gastrointest Endosc 55:655–661
Griffin JM, Reed CE, Denlinger CE (2012) Utility of restaging endoscopic ultrasound after neoadjuvant therapy for esophageal cancer. Ann Thorac Surg 93:1855–1860
Kalha I, Kaw M, Fukami N, Patel M, Singh S, Gagneja H, Cohen D, Morris J (2004) The accuracy of endoscopic ultrasound in restaging esophageal carcinoma after chemoradiation therapy. Cancer 101:940–947
Laterza E, Manzoni G, Guglielmi A, Rodalle L, Tedesco P, Cordianl C (1999) Endoscopic ultrasonography in the staging of esophageal carcinoma after preoperative radiotherapy and chemotherapy. Ann Thorac Surg 67:1466–1469
Zuccaro G, Rice TW, Goldblum J, Medendorp SV, Becker M, Pimentel R, Gitlin L, Adelstein DJ (1999) Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 94:906–912
Lorenzen S, Thuss-Patience P, Al-Batran SE, Lordick F, Haller B, Schuster T, Pauligk C, Luley K, Biches D, Schumacher G, Homann N (2013) Impact of complete pathologic response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol 24:2068–2074
Mesenas S, Vu C, McStay M, Doig L, Mason R, Boyle N, Meenan J (2008) A large series, resection controlled study to assess the value of radial EUS in restaging gastroesophageal cancer following neoadjuvant chemotherapy. Dis Esophag 21:37–42
Misra S, Choi M, Livingstone AS, Franceschi D (2012) The role of endoscopic ultrasound in assessing tumor response and staging after neoadjuvant chemotherapy for esophageal cancer. Surg Endosc 26:518–522
Catalano MF, Sivak MV Jr, Rice T, Gragg LA, van Dam J (1994) Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 40:442–446
Heinzow HS, Seifert H, Tsepetonidis S, Wolters H, Kucharzik T, Doschke W, Domagk D, Meister T (2013) Endoscopic ultrasound in staging esophageal cancer after neoadjuvant chemotherapy—results of a multicenter cohort analysis. J Gastrointest Surg 17:1050–1057
Bohle W, Clemens P, Tran D, Zoller WG (2011) Stellenwert der Endosonografie bei der Therapieplanung des resektablen Ösophaguskarzinoms. Z Gastroenterol 49:1102
Chak A, Canto MI, Cooper GS, Isenberg G, Willis J, Levithan N, Clayman J, Forastiere A, Heath E, Sivak MV (2000) Endosonographic assessment of multimodality therapy predicts survival of esophageal cancer patients. Cancer 88:1788–1795
Ribeiro A, Franceschi D, Parra J, Livingstone A, Lima M, Hamilton-Nelson K, Ardala B (2006) Endoscopic ultrasound restaging after neoadjuvant chemotherapy in esophageal cancer. Am J Gastroenterol 101:1216–1221
Machlenkin S, Lezler E, Ideleich E, Ziv-Sokolovsky N, Klein Y, Kahstan H (2009) Endoscopic ultrasound: doubtful accuracy for restaging esophageal cancer after preoperative chemotherapy. IMAJ 11:166–169
Bowrey DJ, Clark GWB, Roberts A, Hawthorne AB, Maugham TS, Williams GT, Carey PD (1999) Serial endoscopic ultrasound in the assessment of response to chemoradiotherapy for carcinoma of the esophagus. J Gastrointest Surg 3:462–467
Eloubeidi MA, Cerfolio RJ, Bryani AS, Varadaraiulu S (2011) Efficacy of endoscopic ultrasound in patients with esophageal cancer predicted to have N0 disease. Eur J Cardiothorac Surg 40:636–641
Chandawarkar RY, Kakegawa T, Fujita H, Yamana H, Toh Y, Fujitoh H (1996) Endosonography for preoperative staging of specific nodal groups associated with esophageal cancer. World J Surg 20:700–702
Menzel J, Hoepffner N, Nottberg H, Schulz C, Senninger N, Domschke W (1999) Preoperative staging of esophageal carcinoma: miniprobe sonography versus conventional endoscopic ultrasound in a prospective histopathologically verified study. Endoscopy 31:291–297
Giovannini M, Seitz JF, Thomas P, Hannoun-Levy JM, Perrier Resbeut M, Delpero JR, Fuentes P (1997) Endoscopic ultrasonography for assessment of the response to combined radiation therapy and chemotherapy in patients with esophageal cancer. Endoscopy 29:4–9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
W. Bohle, M. Kasper and W. G. Zoller have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Bohle, W., Kasper, M. & Zoller, W.G. Different accuracy of endosonographic tumor staging after neoadjuvant chemotherapy and chemoradiotherapy in esophageal cancer. Surg Endosc 30, 2922–2928 (2016). https://doi.org/10.1007/s00464-015-4578-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4578-y