Abstract
Background
Synchronous gastric neoplasms are not infrequently detected, thus endoscopic submucosal dissection (ESD) for multiple early gastric neoplasia is occasionally considered. However, there have been few investigations of the safety and feasibility of simultaneous ESD for multiple gastric lesions. This study aims to evaluate the safety and feasibility of simultaneous ESD for multiple gastric neoplasia.
Methods
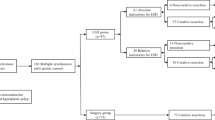
A total of 1823 patients who underwent ESD for 1929 gastric adenomas or early gastric cancers were retrospectively reviewed in this study. Two hundred gastric adenomas or early gastric cancers among 94 patients were treated by ESD simultaneously (multiple group), and 1729 patients were treated with ESD for a single lesion (single group).
Results
En bloc resection (P = 0.060), complete resection (P = 0.362) and curative resection (P = 0.108) rates did not differ between the two groups. Rates of adverse events including bleeding (P = 0.317), perforation (P = 0.316) and aspiration pneumonia (P = 0.563) were not higher in the multiple group. Long-term follow-up showed more frequent local recurrence (P < 0.001), synchronous neoplasia (P = 0.041) and metachronous neoplasia (P < 0.001) per patient in the multiple group; however, local recurrence per lesion did not differ between the two groups (P = 0.103).
Conclusions
Simultaneous ESD for multiple synchronous gastric neoplasms is safe and feasible compared to single ESD. However, thorough examination for local recurrence and synchronous and metachronous neoplasia is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The detection and diagnosis of early gastric cancer has increased due to advances in endoscopic examination and endoscopic screening. Endoscopic resection as a minimally invasive therapy has been widely accepted in Asian countries, including Korea and Japan, for cases of gastric neoplasia that are confined to the mucosa and have little evidence of lymph node metastasis [1–3]. In particular, endoscopic submucosal dissection (ESD) is widely used because it allows for a single slice resection of gastric lesions, regardless of tumor size [4, 5].
Two or more malignant foci in the stomach are often diagnosed during endoscopy and are known as synchronous multifocal gastric cancer. The multifocality or multicentricity rate of early gastric cancer (EGC) has been reported to range from 4.8 to 20.9 % at several institutions [6–8]. Recent studies have shown that multiple synchronous EGCs have clinicopathologic features and lymph node metastasis risk similar to those of solitary EGCs [9, 10]. Therefore, endoscopic treatment could be feasible when major and minor lesions are predicted to represent mucosal cancer without lymphovascular invasion.
However, ESD is a time-consuming procedure that requires great endoscopic skill [4, 11]. Simultaneous ESD for multiple gastric neoplasms would increase procedure time and the amount of resected mucosa, which may increase the risk of complications and unfavorable outcomes. On the other hand, separate procedures with a time interval between each gastric lesion would result in a longer period of hospitalization and increase medical expense. To the best of our knowledge, there have been few studies evaluating adverse events, feasibility or outcomes related to ESD for multifocal gastric neoplasms. A recent study performed in Japan reviewed relatively small number of patients and did not evaluate long-term outcomes [12]. Our study aimed to evaluate the safety, feasibility and outcomes of simultaneous ESD for multiple synchronous gastric neoplasia and compare it to that of ESD for solitary gastric lesion.
Materials and methods
Patients
Patients with gastric neoplasia who underwent ESD were prospectively followed at a single tertiary teaching hospital in Seoul, Korea, from January 2008 to December 2011. Clinical data included patient demographics, pathologic data of gastric neoplasms, results of endoscopic resection and procedure-related adverse events including bleeding and perforation. A total of 94 patients underwent simultaneous ESD for a total of 200 synchronous EGCs or gastric adenomas. They were compared to 1729 patients who underwent ESD for single gastric neoplasm. Gastric neoplasms included in the study were selected based on the expanded criteria proposed by Gotoda et al. [3]. The patients in the multiple group were treated by ESD under a single anesthesia on a single day. Patients who had prior gastric resection were excluded. The institutional review board of the hospital approved this study.
Study definitions
The macroscopic type and location of EGC were classified according to the Japanese Gastric Cancer Association classification system [13]. En bloc resection was defined as resection in a single piece as opposed to resection of multiple pieces. Complete resection was defined as tumor-free lateral and vertical margins on pathologic examination. Curative resection was defined as en bloc and complete resection without submucosal invasion deeper than 500 μm from the muscularis mucosae, lymphatic invasion and vascular involvement. The main lesion was defined as being histologically more advanced, and larger, if the histology was the same. The accessory lesion was defined as histologically less advanced, and smaller, if histology was the same. The diameter of the lesion was defined as the longest diameter of the neoplasm measured in the resected specimen on pathologic examination. Procedure time was defined as the time from marking of mucosa to complete removal, including the time required for hemostasis. Bleeding was defined as (A) intraoperative bleeding that required blood transfusion, (B) clinical symptoms such as melena or hematemesis or (C) a decrease in hemoglobin level >2 g/dL following procedure. A diagnosis of perforation required direct endoscopic visualization of mesenteric fat or radiographic evidence of free air. Pneumonia was defined as new or progressive consolidation with one of the following newly developed criteria: (A) cough, (B) purulent sputum or change in character of sputum or (C) rales or dullness to percussion on physical examination of the chest [14]. Parenteral administration of nonsteroidal anti-inflammatory drugs or opioid analgesics was analyzed as the number of pain killer injections. Complete blood cell counts measured at the day of hospitalization and the day after ESD were retrospectively reviewed.
ESD methods
Endoscopic procedure was done with single channel endoscope with jet function available (GIF Q260J or GIF-H260Z, Olympus Optical Co. Ltd., Tokyo, Japan). After endoscopic evaluation of the gastric lesions with indigo carmine stain, the surrounding lesion was marked by electrocautery (ICC 200; ERBE, Tübingen, Germany) using an argon plasma coagulation probe or a needle knife (KD-10Q-1-A, Olympus Optical Co. Ltd., Tokyo, Japan). Saline mixed with epinephrine (0.01 mg/mL) and 0.8 % indigo carmine was injected into the submucosa to lift the lesion. A circumferential incision (precut) was made along the outer border of the lesion using a needle knife and an insulated-tipped knife (IT knife, KD-610L, Olympus Optical Co. Ltd., Tokyo, Japan). The submucosal layer was then dissected with the IT knife until complete removal was achieved. Endoscopic hemostasis was performed with a hemoclip or hemostatic forceps for bleeding or an exposed vessel. For multiple synchronous lesions, marking was performed for all lesions initially. After complete dissection and hemostasis of the first lesion, an epinephrine mixture was injected, and a precut was made subsequently for the residual neoplasms.
Follow-up
For EGCs, EGD was scheduled at 3, 6, 12, 18 and 24 months after ESD to check for local or metachronous lesions. After 24 months, EGD was performed annually. For adenomas, EGD was performed at 3, 12 months after ESD and annually thereafter.
Recurrent neoplasia detected at the curatively resected site was regarded as local recurrence. A second neoplasm detected at the gastric site other than the primary resection area within 12 months after endoscopic resection was defined as synchronous. A second neoplasm found at sites other than the primary resection area at 12 months or later was defined as metachronous.
Statistical analysis
For statistical analysis, we used χ 2 test, Fisher’s exact test and t test. The Kaplan–Meier method and a log-rank test were used for survival analysis of long-term outcomes. A P value < 0.05 was regarded as a significant difference for group comparisons. Statistical analysis was performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of patients
A total of 1823 patients who had undergone endoscopic resection for 1929 gastric adenomas or early gastric cancers were enrolled in this study. Ninety-four patients had two or more gastric lesions. The rate of simultaneous ESD for synchronous gastric neoplasia was 5.16 % (94 out of 1823). The baseline characteristics of patients who underwent endoscopic resection are shown in Table 1. The mean age was greater in patients with multiple lesions (67.03 ± 7.35 vs. 63.28 ± 9.44, P < 0.001). A history of cigarette smoking was more common (61.7 vs. 47.8 %, P = 0.009), and underlying comorbid diseases, including cardiovascular disease, renal disease, diabetes, and chronic viral hepatitis, were more frequent in patients with multiple lesions (62.8 vs. 48.0 %, P = 0.005). Other baseline characteristics of the patients did not differ between the two groups.
Comparison of clinicopathologic characteristics of gastric neoplasms
In the multiple groups, 12 patients had triple lesions and the remaining 82 had double lesions. Morphologic and pathologic characteristics of the main lesions, which were histologically more advanced or larger when histology was the same, were compared to those of the solitary lesions. The mean diameter of the main lesion of the multiple group was significantly longer than that of the single group (15.24 ± 9.89 vs. 12.90 ± 9.30 mm, P = 0.020). Histology, shape and location were comparable between the two groups (Table 2). When the main lesion was compared to the accessory lesions of the multiple group, shape and location did not differ (Table 3).
Comparison complications and morbidities
Table 4 shows a comparison of the procedure time, adverse events and variables related to morbidity in each group. The mean procedure time was longer in the multiple group (94.99 ± 48.96 vs. 57.91 ± 42.73 min, P < 0.001), but adverse events including bleeding, perforation and aspiration pneumonia did not differ between the two groups. The mean number of hospital days and pain killer injections was not significantly different between the two groups. Also, the decrement of serum hemoglobin level was not significantly different between the two groups; however, the increment of white blood cell (WBC) count was larger in the multiple ESD group (4335 ± 2694 vs. 3725 ± 2610/µL, P = 0.034).
Procedural outcomes and long-term outcomes
As shown in Table 5, the procedural outcome including en bloc resection, complete resection and curative resection did not differ between the two groups.
Patients who were curatively resected and who had a follow-up period longer than 1 year were analyzed for long-term outcome. In total, 1184 patients for single group and 54 patients for multiple group were analyzed, and the median follow-up periods were 27 months [interquartile range (IQR) 18.3–36.3 months] and 19 months (IQR 13.0–24.2 months), respectively. The cumulative local recurrence (P < 0.001), cumulative incidence of synchronous neoplasia (P = 0.041) and metachronous neoplasia (P < 0.001) were higher in the multiple group. Among five cases of local recurrence in the multiple neoplasms, four occurred at the main lesion. However, when the cumulative incidence of local recurrence was considered per resected lesion, not per patient, there was no difference in the two groups (P = 0.103) (Fig. 1).
Kaplan−Meier plot for long-term outcomes. A Cumulative incidence of local recurrence per patient and B per resected lesion. C Cumulative incidence of synchronous neoplasia and D metachronous neoplasia. Curatively resected patients who had a follow-up period longer than 1 year were analyzed for long-term outcomes. A total of 1184 patients from the single ESD group and 54 patients from the multiple ESD group were analyzed, and the median follow-up period was 27 months (IQR of 18.3–36.3 months) and 19 months (IQR of 13.0–24.2 months), respectively. ESD, endoscopic submucosal dissection; IQR, interquartile range
Figure 2 shows that the estimated disease-free survival was significantly longer in the single group compared to the multiple group (45.41 ± 0.32 vs. 38.90 ± 2.45 months, P < 0.001).
Discussion
Our study is the largest study to demonstrate the safety and feasibility of simultaneous ESD for multiple lesions as compared to ESD for a single lesion. A previous study evaluating safety and efficacy of simultaneous ESD for synchronous double EGCs only included double lesions (not triple), a relatively small number of patients, and there was no analysis of long-term outcomes [12].
In this study, comparison of the single group and the multiple group revealed no significant difference in adverse events including bleeding, perforation and aspiration pneumonia. Also, patients in the multiple group showed neither longer hospitalization nor more pain killer injections. Review of the resected specimens showed no significant difference in the rate of en bloc resection, complete resection or curative resection. Our findings demonstrate that the technical safety and feasibility of simultaneous ESD for multiple gastric neoplasms are acceptable compared to ESD for a single neoplasm. On the other hand, metachronous recurrence after endoscopic resection in the multiple group was significantly higher than that in the single group.
In this present study, baseline characteristics showed that patients in the multiple group were older and more likely to have comorbidities than the patients in the single group. This corresponds to the previous studies reporting old age as one of the risk factors for multifocality in gastric neoplasms [8]. Frequent comorbidities in the multiple group may be ascribed to older age, since more comorbidities are generally expected in the elderly.
As expected, the procedure time was significantly longer in the multiple group. However, the mean procedure time for simultaneous ESD was not twice that of single ESD procedures. This may be due to ease of ESD of accessory lesions compared to the main lesion resulting from less advanced histology and smaller diameter.
Longer procedure time and poor visual field due to the previously resected specimen or blood clots may interfere with the resection of second or third target lesions. Nevertheless, our data did not show evidence of frequent adverse events such as bleeding, perforation or aspiration pneumonia related to simultaneous ESD. The rate of perforation was remarkably high in the multiple group (4.3 vs. 2.6 %), but did not reach statistical significance. The majority of perforation events were minimal and conservatively managed (39 out of 45 cases in the single group and all 4 cases in the multiple group). However, the increment of WBC count was significantly higher in the multiple ESD group. There were reports describing large size and long procedure time as risk factors for adverse events [15, 16]. A previous study performed in our institute demonstrated a procedure time of more than 2 h as a risk factor for aspiration pneumonia during ESD [17]. Moreover, a recent study performed in Japan indicated that procedure time longer than 150 min is an independent predictor of adverse events in simultaneous ESD for double EGC [12]. Though the mean procedure time did not reach 2 h in our study, ESD took longer for the multiple group than for the single group, which may be related to minor events of aspiration and pulmonary infection. Moreover, a larger amount of resected mucosa and mucosal injury from electrocauterization may account for inflammatory reactions which can lead to leukocytosis.
The mean number of hospital days was greater in the multiple group, but the difference was not statistically significant. Also, patients in the multiple group received more parenteral pain killer injections after the procedure, but this difference was also not statistically significant. According to our results, it is unlikely that simultaneous ESD would cause more medical expense or morbidity. Rather, separate performance of endoscopic procedures would prolong hospitalization and consequently cause more medical expenses.
On long-term follow-up, local recurrence was more frequent in the multiple group than the single group. However, considering the risk of recurrence per lesion, the risk of local recurrence may well be increased with multiple lesions. In fact, the cumulative incidence of local recurrence per resected lesion did not differ between the two groups (P = 0.103). Therefore, more thorough examination and biopsies of each resected site during follow-up are important.
Reported overall incidence rates of metachronous gastric cancer after endoscopic resection range from 7.9 to 14 % [18–22]. As for predictive factors, synchronous multiplicity of the gastric cancer and patient age at the time of the initial endoscopic resection has been reported to significantly affect the incidence of metachronous lesions [20]. Our results, which showed a higher incidence of synchronous neoplasia as well as metachronous neoplasia in the multiple group, are consistent with the results from the previous report. However, this finding was not adjusted by known risk factor for metachronous neoplasia such as H. pylori status and extent of atrophy due to limited available data [23, 24].
The main limitation of this study is that it was a retrospective single-center study. However, the number of patients included in this study was large, and most of the data used for this study were collected prospectively for future analysis, so there was a little chance of bias. Future studies with a larger number of cases and a longer follow-up period would be useful to verify our results.
In conclusion, simultaneous ESD of multiple gastric neoplasms is safe, feasible and may reduce overall medical expense compared to multiple ESD separated by time intervals. However, in order to maintain optimal outcome, thorough examination for local recurrence, synchronous and metachronous neoplasia is essential.
References
Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, Sasako M, Sano T, Ochiai A, Yoshida S (1996) Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer 77(4):602–606. doi:10.1002/(SICI)1097-0142(19960215)77:4<602:AID-CNCR3>3.0.CO;2-I
Yasuda K, Shiraishi N, Suematsu T, Yamaguchi K, Adachi Y, Kitano S (1999) Rate of detection of lymph node metastasis is correlated with the depth of submucosal invasion in early stage gastric carcinoma. Cancer 85(10):2119–2123
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3(4):219–225
Gotoda T, Yamamoto H, Soetikno RM (2006) Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 41(10):929–942. doi:10.1007/s00535-006-1954-3
Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, Yoshida M, Ohkuwa M, Hosokawa K, Tajiri H, Yoshida S (2002) A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc 55(4):576–581
Honmyo U, Misumi A, Murakami A, Haga Y, Akagi M (1989) Clinicopathological analysis of synchronous multiple gastric carcinoma. Eur J Surg Oncol 15(4):316–321
Kim HG, Ryu SY, Lee JH, Kim DY (2012) Clinicopathologic features and prognosis of synchronous multiple gastric carcinomas. Acta Chir Belg 112(2):148–153
Otsuji E, Kuriu Y, Ichikawa D, Okamoto K, Hagiwara A, Yamagishi H (2005) Clinicopathologic characteristics and prognosis of synchronous multifocal gastric carcinomas. Am J Surg 189(1):116–119. doi:10.1016/j.amjsurg.2004.03.013
Kim HM, Kim HK, Lee SK, Cho JH, Pak KH, Hyung WJ, Noh SH, Kim CB, Lee YC, Song SY, Youn YH (2012) Multifocality in early gastric cancer does not increase the risk of lymph node metastasis in a single-center study. Ann Surg Oncol 19(4):1251–1256. doi:10.1245/s10434-011-2083-7
Choi J, Kim SG, Im JP, Kang SJ, Lee HJ, Yang HK, Kim JS, Kim WH, Jung HC, Song IS (2011) Lymph node metastasis in multiple synchronous early gastric cancer. Gastrointest Endosc 74(2):276–284. doi:10.1016/j.gie.2011.04.009
Akasaka T, Nishida T, Tsutsui S, Michida T, Yamada T, Ogiyama H, Kitamura S, Ichiba M, Komori M, Nishiyama O, Nakanishi F, Zushi S, Nishihara A, Iijima H, Tsujii M, Hayashi N (2011) Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc 23(1):73–77. doi:10.1111/j.1443-1661.2010.01062.x
Kasuga A, Yamamoto Y, Fujisaki J, Okada K, Omae M, Ishiyama A, Hirasawa T, Chino A, Tsuchida T, Hoshino E, Igarashi M (2012) Simultaneous endoscopic submucosal dissection for synchronous double early gastric cancer. Gastric Cancer. doi:10.1007/s10120-012-0218-6
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112. doi:10.1007/s10120-011-0041-5
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections. Am J Infect Control 16(3):128–140
Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, Morikawa T, Murakami T, Tomoda J (2012) Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol 27(5):907–912. doi:10.1111/j.1440-1746.2011.07039.x
Ohta T, Ishihara R, Uedo N, Takeuchi Y, Nagai K, Matsui F, Kawada N, Yamashina T, Kanzaki H, Hanafusa M, Yamamoto S, Hanaoka N, Higashino K, Iishi H (2012) Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest Endosc 75(6):1159–1165. doi:10.1016/j.gie.2012.02.015
Park CH, Kim H, Kang YA, Cho IR, Kim B, Heo SJ, Shin S, Lee H, Park JC, Shin SK, Lee YC, Lee SK (2012) Risk factors and prognosis of pulmonary complications after endoscopic submucosal dissection for gastric neoplasia. Dig Dis Sci. doi:10.1007/s10620-012-2376-0
Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D (2006) Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer 9(2):93–98. doi:10.1007/s10120-006-0372-9
Uemura N, Okamoto S (2000) Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer in Japan. Gastroenterol Clin N Am 29(4):819–827
Arima N, Adachi K, Katsube T, Amano K, Ishihara S, Watanabe M, Kinoshita Y (1999) Predictive factors for metachronous recurrence of early gastric cancer after endoscopic treatment. J Clin Gastroenterol 29(1):44–47
Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I (2005) Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy 37(10):990–993. doi:10.1055/s-2005-870198
Kobayashi M, Narisawa R, Sato Y, Takeuchi M, Aoyagi Y (2010) Self-limiting risk of metachronous gastric cancers after endoscopic resection. Dig Endosc 22(3):169–173. doi:10.1111/j.1443-1661.2010.00987.x
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M (2008) Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 372(9636):392–397. doi:10.1016/S0140-6736(08)61159-9
Hanaoka N, Uedo N, Shiotani A, Inoue T, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Haruma K, Tatsuta M (2010) Autofluorescence imaging for predicting development of metachronous gastric cancer after Helicobacter pylori eradication. J Gastroenterol Hepatol 25(12):1844–1849. doi:10.1111/j.1440-1746.2010.06442.x
Disclosures
Dong Hoo Joh, Chan Hyuk Park, Sungmo Jung, Seung-Ho Choi, Hyun Ki Kim, Hyuk Lee, Jun Chul Park, Sung Kwan Shin, Yong Chan Lee, Sang Kil Lee have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joh, D.H., Park, C.H., Jung, S. et al. Safety and feasibility of simultaneous endoscopic submucosal dissection for multiple gastric neoplasias. Surg Endosc 29, 3690–3697 (2015). https://doi.org/10.1007/s00464-015-4139-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4139-4