Abstract
Background
Patients with early gastric cancer (EGC) are at high risk of developing synchronous multiple gastric neoplasms (SMGNs) after undergoing endoscopic submucosal dissection (ESD). However, most previous studies have had small sample sizes, and few have focused on association studies.
Aims
This study aimed to analyze the associations between SMGN lesion data from patients with EGC treated with ESD and their correlation coefficients.
Methods
The clinical ESD data from two hospitals from January 2008 to January 2021 were retrospectively analyzed. The main lesions were defined as those with a significant depth of infiltration. The larger tumor diameter was considered the main lesion if the lesions had the same infiltration depth.
Results
Of the 1013 post-ESD cases examined, 95 cases (223 lesions) had SMGN, and 25 patients had more than three lesions. For the correlation analysis, 190 lesions were included. The study revealed a similarity in pathological type between main and minor lesions (rs = 0.37) and a positive correlation in infiltration depth (rs = 0.58). The mean diameter sizes of the main and minor lesions were 20.7 ± 8.3 mm and 13.1 ± 6.4 mm, respectively, with statistically significant differences (P < 0.001). A linear correlation was observed between the diameter size and a linear regression model was constructed, producing r = 0.38 [95% confidence interval (CI) 0.19–0.54], b = 0.29 (95% CI 0.14–0.44), t = 3.94, P < 0.001]. A correlation was identified between the vertical distribution of the main and minor lesions, the horizontal distribution, and the gross endoscopic morphology (ϕc = 0.25, P = 0.02; ϕc = 0.32, P < 0.001; ϕc = 0.60, P < 0.001).
Conclusions
The correlation coefficients for microscopic characteristics were higher than those for gastroscopy. There is a significant positive correlation between the main and minor lesions regarding pathological stage and depth of infiltration, respectively. The spatial distribution of the lesions and the gastroscopic morphology were similar.
Graphical abstract
SMGN synchronous multiple gastric neoplasms, ESD endoscopic submucosal dissection.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is one of the most common malignancies worldwide. Early gastric cancer (EGC) is cancer cells confined to the mucosal or submucosal layer, with or without lymph node metastases. Fortunately, the prognosis for EGC is generally favorable, with a 5-year survival rate of more than 90% [1]. Unlike surgery, endoscopic submucosal dissection (ESD) is a minimally invasive gastrointestinal endoscopic technique that preserves the normal physiological functioning of the entire stomach. ESD has shown a prognosis comparable to surgery and has become the standard for treating EGC [2]. However, researchers have identified multiple histopathological occurrences in postoperative cases of gastric cancer [3]. While preserving the entire gastric mucosa, ESD raises concerns about the event of postoperative synchronous tumors or the potential for missed synchronous tumors [1, 4]. Two or more EGC lesions detected either before the initial ESD or within 1 year after ESD are referred to as simultaneous multiple early gastric cancers (SMEGC).
Consequently, exploring SMEGC has become a prominent research topic in recent years. Various studies have been conducted to analyze risk factors contributing to missed diagnoses, risk of lymph node metastasis, pathological characteristics, prognosis of the disease, and genomic studies associated with SMEGC [5,6,7]. However, it should be noted that specific study cohorts have been derived from surgical cases rather than cases involving ESD. Furthermore, the incidence of SMEGC is low, accounting for approximately 9–14% of all gastric cancer cases [8]. Many existing studies focusing on SMEGC face limitations due to the small sample sizes (n < 40) and the limited availability of statistical data. It is worth noting that studies on multifocal correlations are rare. Early studies focused mainly on descriptive statistics [9]. Due to the limited sample size, there are several subgroups with a sample size of 0, which introduces bias and reduces the credibility of the findings [10]. Furthermore, the existing studies did not assess the strength of the correlation between the main and minor lesions, and the data on the correlation coefficient remain unknown. Consequently, the results of the previous studies are not comprehensive, highlighting the need for a larger sample size to validate the findings and obtain additional correlation coefficients.
There is a significant likelihood of intraepithelial neoplasia progressing to gastric cancer in the near term, thus justifying its inclusion as an indication for ESD [11]. Our study enrolled patients with synchronous multiple gastric neoplasms (SMGN), including SMEGC and intraepithelial neoplasia [5]. Expanding the sample size will allow for more robust analysis and validation of the findings. On the other hand, conducting more in-depth research on SMEGC and early lesions will provide a deeper understanding of the disease and its characteristics. This study aimed to analyze the correlation between SMGN lesions and summarize the association between various factors. Additionally, data on correlation coefficients between the main and minor lesions were included in the study. These additional data provide valuable information on the relationship between these lesions in SMGN patients. By incorporating correlation coefficients, researchers can quantitatively assess the strength and direction of the correlation, improving the understanding of the disease.
Materials and methods
Study design and definitions
This retrospective analysis was conducted at two hospitals in China, the Fujian Provincial Hospital South Branch, and the Fujian Provincial Hospital, Fuzhou, from January 2008 to January 2021. Two or more lesions detected either before the initial ESD or within 1 year after ESD, including SMEGC and intraepithelial neoplasia, are called SMGN. The inclusion criteria were patients who received ESD and who were pathologically confirmed as SMGN. The diagnosis of SMGN was based on Moertel criteria [12]. These criteria consists of the following components: (1) pathological examination that confirmed each lesion as tumors or intraepithelial neoplasia, (2) microscopic observation of normal gastric mucosa between different lesions, and (3) exclusion of lesions resulting from local infiltration or metastasis. Basic information, endoscopic variables, and postoperative pathological variables were recorded for each case. Gastroscopy was performed using a visual gastroscope (GF-H260; Olympus Optical Co., Ltd., Tokyo, Japan). At least two endoscopists with at least 10 years of experience in endoscopy reviewed the gastroscopic findings. Gastroscopy reports were obtained from the hospital’s endoscopy database. The study protocol was approved by the hospital’s ethical review committee (approval number K2021-04-035, approval date April 15, 2021).
Moertel’s criteria were used to classify major and minor lesions as follows: (1) in cases where two or more lesions had the same depth of infiltration, the lesion with the largest diameter was designated as the major lesion, while the remaining lesions were classified as minor lesions; and (2) if two or more lesions exhibited different depths of infiltration, the lesion with the highest depth of infiltration was classified as the major lesion, while the others were categorized as minor lesions. In cases with more than three EGC lesions, the secondary main lesion was considered a minor lesion.
The gross gastroscopic morphology of SMGN was classified into three groups according to the Paris typology [13]. Superficial gastric cancer (Type 0) is classified into three categories: elevated lesions (Type 0-I), flat lesions (Type 0-II), and depressed lesions (Type 0-III). Within Type 0-I, there are two subdivisions: pedunculated type (Type 0-Ip) and non-pedunculated type (Type 0-Is). Type 0-II is divided into three subtypes: Type 0-IIa, Type 0-IIb, and Type 0-IIc, based on the degree of slight elevation, flatness, and slight depression of the lesions. The cutoff between Type 0-I and Type 0-IIa is a bulge height of 2.5 mm (measured as the closed thickness of the biopsy forceps), and the cutoff between Type 0-III and Type 0-IIc is a depression depth of 1.2 mm (measured as the open thickness of the biopsy forceps with a single clamp). The macroscopic type of tumor was divided into three types: elevated (0-I, 0-IIa), flat (0-IIb), and depressed (0-IIa+IIc, 0-IIc, 0-III).
Pathologically, the lesions were divided into three groups based on the WHO classification criteria [14]: precancerous lesions (intraepithelial neoplasia), differentiated adenocarcinoma (well and moderately differentiated tubular adenocarcinomas), and undifferentiated tubular adenocarcinomas (poorly differentiated tubular adenocarcinoma, signet-ring cell carcinoma, and poorly cohesive carcinoma). The depth of infiltration of the early lesions was categorized into three categories: mucosal lamina, mucosal muscularis, and submucosa. Based on the microscopic presentation, background mucosal atrophy and intestinal metaplasia were classified as mild, moderate, or severe. The horizontal distribution of the tumor was classified as the anterior wall, the lesser curvature, the posterior wall, and the greater curvature. The vertical distribution was divided into the upper (cardia, fundus), middle (gastric body), and lower (gastric angle, sinus, and pylorus) regions [14, 15].
Early ESD guidelines were differentiated-type adenocarcinoma without ulcerative findings, of which the depth of invasion is clinically diagnosed as T1a and the diameter is ≤ 2 cm [16]. The latest edition of the ESD guidelines integrates a significant portion of expanded indications into the category of absolute indications. The absolute indications for ESD were (1) differentiated mucosal endocarcinoma without ulcers, (2) differentiated mucosal endocarcinoma with ulcers and lesions ≤ 3 cm, (3) high-grade intraepithelial neoplasia of the gastric mucosa, and (4) undifferentiated mucosal endocarcinoma with lesions ≤ 2 cm and no ulcers [11]. Endoscopic examinations are conducted at 3 months, 6 months, and 1 year postoperatively.
Statistical analysis
Statistical analysis was conducted using R (version 4.2.3, R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org) and SPSS 25.0 (SPSS Inc. Chicago, Illinois, USA). Normally distributed data are reported as mean ± standard deviation, while count data are presented as frequencies and percentages (%). The Chi-square test or Fisher’s exact test was used for group comparisons. The Spearman test was used to analyze the association of grade variables, while the Cramer V test was utilized to investigate the association of unordered multicategorical variables. Linear regression analysis was employed to determine the correlation between the sizes of main and minor lesions in SMGN patients. P values < 0.05 were considered statistically significant.
Results
Clinical characteristics
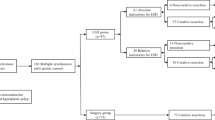
A total of 1031 patients with EGC who were followed up 12 months after ESD, and 936 were excluded (899 cases of solitary cancer and 37 cases with various other types of gastric tumors). Among the 95 (9.4%) patients diagnosed with SMGN (223 lesions after surgery), 25 patients (26.3%) had more than three lesions. The median time to detection of the synchronous multiple lesions was 7.4 months (range 3.9–11.3). The analysis included the final data of 190 lesions. Thirty-three lesions other than the main and minor ones were excluded as they were the third or subsequent lesions within the same patient (Fig. 1). The mean age was 63.9 ± 7.4 years, and 74 (77.9%) were men. The Helicobacter pylori infection rate was 52.6%. The baseline characteristics of the patients are shown in Table 1.
The gastroscopic similarity of the main and minor lesions
Table 2 presents the statistical analysis of the spatial distribution areas and endoscopic macrotypes of the main and minor lesions in SMGN. Most main and minor lesions exhibited significant similarity in spatial distribution areas and the endoscopic macrotypes. In particular, many main and minor lesions in SMGN were found in the distal stomach. Specifically, 55.1% of the minor lesions were also observed in the lower part of the stomach when the main lesion was located in the lower part. In the upper part of the stomach, 60% (9/15) of minor lesions were located in the same region as the main lesion. Similarly, when the lesions were located in the in the middle or lower area of the stomach, 32.3% (10/31), 55.1% (27/49) of the minor lesions were also located in the same region as the main lesion. Overall, 48.4% of the lesions were distributed in the same vertical position. Likewise, when the lesions were located in the anterior wall, posterior wall, lesser, and greater curvatures of the stomach, 46.2%, 59.3%, 42.1%, and 5.9% of the minor lesions were found in the same region as the main lesion, respectively, in the corresponding horizontal positions. Consequently, 41.1% of the lesions were distributed in the same horizontal position. Among the lesions analyzed, 62.5% were of the elevated type, 64.7% were of the flat type, and 87.0% were classified as depressed. This indicates that 76.8% of the lesions exhibited the same endoscopic macrotypes. The Cramer V test examined the correlation between the spatial distribution of the lesions and the endoscopic macrotypes. The results showed significant correlations between the horizontal distribution (χ2 = 27.47, ϕc = 0.32, P < 0.001) (Fig. 2A), vertical distribution (χ2 = 11.62, ϕc = 0.25, P = 0.02) (Fig. 2B), and endoscopic macrotypes (χ2 = 53.80, ϕc = 0.60, P < 0.001) (Fig. 2C) of the main and minor lesions.
Cramer’s V correlation heat map. Data types are unordered multi-categorical variables, using Cramer’s V test. Vertical distribution. ϕc = 0.25, P = 0.02 (A). Horizontal distribution. ϕc = 0.32, P < 0.001 (B). Gross endoscopic morphology. ϕc = 0.60, P < 0.001 (C). There is a correlation between the spatial distribution of the main and minor lesions or the gross endoscopic morphology
Correlation heat map
A positive correlation was observed between the diameters of the main and minor lesions and the depth of lesion infiltration (rs = 0.48, rs = 0.58). Furthermore, there was a similarity in the pathology type between the main and minor lesions (rs = 0.37). A positive correlation was observed between the lesion size and the severity of the pathology type, the degree of mucosal background atrophy, and the degree of intestinal metaplasia (rs = 0.30, rs = 0.33). All these results were statistically significant. In contrast, no association was found between sex, age, gastric mucosal background, lesion characteristics, or pathological features (Fig. 3).
Heat map of the correlations of main and minor lesions. There was similarity in pathological type between the main and minor lesions (rs = 0.37); a positive correlation between infiltration depth or lesion diameter (rs = 0.58, rs = 0.49); a positive correlation between lesion size and pathological type or degree of intestinal metaplasia of mucosal background atrophy (rs = 0.30, rs = 0.33). No association was found between gender, age, gastric mucosal background, lesion characteristics, or pathological features. *P < 0.05, **P < 0.01, ***P < 0.001
Linear regression analysis of the diameters of the main and minor lesions
The mean diameter sizes of the main and minor lesions were 20.7 ± 8.3 mm and 13.1 ± 6.4 mm, respectively, and these values showed statistically significant differences (P < 0.001). To further analyze the relationship between the size of the main and minor lesions, Pearson’s correlation analysis and linear regression analysis were performed to construct regression models (Fig. 4). The results revealed a moderate linear correlation in tumor size between main and minor SMGN lesions, with an r-value of 0.38 [95% confidence interval (CI) 0.19–0.54]. The linear regression model yielded a slope (b) of 0.29 (95% CI 0.14–0.44) and a t-value of 3.94 (P < 0.001).
Discussion
This study contributes to understanding the association between SMGN lesions in patients with ESD. Including a larger sample size and focusing on ESD cases provides valuable information on the correlation between the clinical and pathological characteristics of SMGN. Findings on the association between main and minor lesion size, spatial distribution, endoscopic macrotypes, and microscopic factors, such as pathological features and depth of infiltration, contribute to the knowledge of SMGN. Given the rarity of SMGN and the limited number of studies focused on ESD lesions, this research fills a crucial gap in the literature. By expanding the understanding of the association between SMGN lesions, this study provides essential information for clinical practice.
From a gastroscopic diagnostic point of view, we have identified correlations between lesion size, gross gastroscopic morphology, and spatial distribution. Figure 5 shows a typical case. These findings are consistent with a study conducted by Kim et al., who analyzed 37 patients with SMEGC after surgical and ESD procedures and observed similarities between lesions [10]. However, our study addressed some limitations in their analysis by examining the association between these factors in more detail. We also conducted a detailed correlation analysis, calculating correlation coefficients that had not been previously analyzed. We speculate that progressive gastric cancer can develop by merging adjacent SMGN with the main and minor lesions. Currently, two theories are proposed to explain this phenomenon. The first theory is the “tumor collision” hypothesis, which suggests that a single progressive gastric cancer arises from the horizontal or vertical fusion of several adjacent lesions. The second theory is the “regional carcinogenesis” theory, which proposes that the gastric mucosa is exposed to a pathogenic environment, simultaneously developing carcinoma in multiple neighboring or distant sites [17]. The pattern observed in SMGN lesions, and SMEGC is strikingly similar, highlighting the importance of closely examining the surrounding mucosa for similar morphological characteristics when detecting EGC lesions during gastroscopy. This approach could reduce the likelihood of missed diagnoses. Numerous studies have investigated factors contributing to missed diagnoses of SMEGC, revealing that small flat lesions and lesions in the upper part of the stomach are particularly prone to be overlooked [4, 18]. Our study identified a significant difference in size between main and minor lesions, indicating a higher probability of missing minor lesions within the SMGN, especially smaller lesions located in the upper part of the stomach. A multicenter retrospective cohort study emphasized that lack of endoscopic experience also contributed to missed diagnoses of multiple carcinomas [19].
Endoscopic presentation of main and minor SMGN lesions. In this older woman, a gently elevated lesion with a central depression is observed in the posterior and anterior walls of the gastric sinus. Regional disorganization of the glandular ducts is seen under ME-NBI, with a tortuous extension of the microvasculature and demarcation from the background mucosa (A–C). The lesion was marked by gastroscopy and resected by ESD (D). Postoperative pathology (H&E ×200) of the posterior and anterior walls suggested severe atypical hyperplasia (E, F). SMGN synchronous multiple gastric neoplasms, ME-NBI magnifying endoscopy-narrow band imaging, ESD endoscopic submucosal dissection
From a pathological diagnostic perspective, we observed a stronger correlation in microscopic characteristics between the main and minor lesions. There is a significant positive correlation between the main and minor lesions in terms of pathological stage and depth of infiltration, respectively (Fig. 5). This could be attributed to the gastric mucosa being exposed to a shared physical and chemical environment. The theory of ‘regional carcinogenesis’ can also elucidate this phenomenon. Microscopy serves as the ‘gold standard’ for diagnosing this disease, and the data we obtained were more reliable and comprehensive, with reduced bias, which could lead to stronger correlation coefficients among the lesions [17]. However, it should be noted that our cohort had only three cases of undifferentiated carcinoma and five instances of infiltrating submucosa, which limits our ability to draw definitive conclusions about these cases. Future studies should aim to collect more samples to further explore these specific scenarios.
Substantial evidence suggests that SMGN have earlier staging and better differentiation than single cancers. Furthermore, Takeuchi et al. demonstrated that multiple gastric cancers in surgically treated patients tended to exhibit good differentiation but were prone to co-occurring with other systemic tumors [20]. Wittekind et al. conducted a study involving 1664 patients with gastric cancer and reported that multiple cancers exhibited earlier staging and better pathological differentiation compared to single cancers [21]. Our finding supports that SMGNs tend to have more favorable disease progression and differentiation characteristics. Zhao et al. reported that SMGC was observed more frequently in patients with EGC than in those with advanced gastric cancer [6]. These suggest that SMGC may be more common in the early stages of gastric cancer and further highlight the importance of thorough examination and detection of multiple lesions, particularly in patients with EGC. The hypothesis that multiple cancer lesions may fuse in advanced tumor stages, resulting in a higher prevalence of SMGN in the early stages of the tumor, is an interesting one. The timing of detection and intervention may play a role in the formation and development of SMGN. The study by Seo et al. supports the idea that multifocal carcinomas, particularly undifferentiated adenocarcinomas, are more likely to develop into SMGN [22]. If the disease is not detected early, various lesions can develop and merge, making it difficult to diagnose the disease in time, contributing to its rarity. By closely monitoring these patients, healthcare providers can intervene quickly and provide appropriate treatment strategies to improve patient outcomes.
Our study observed a positive correlation between the degree of background mucosal atrophy and intestinal metaplasia in patients with SMGN. However, we did not find significant associations between intestinalization of mucosal atrophy and factors such as age and sex in this population. Although gastric mucosal atrophic and intestinal metaplasia is typically associated with age and H. pylori infection, it is possible that patients with SMGN have similar degrees of gastric atrophic and intestinal metaplasia, with no differences found between sex and age. The prevalence of H. pylori infection in SMGN in this study was 52.4%. However, some studies suggest that H. pylori infection is not an independent risk factor for SMGN and does not show a statistically significant difference in the H. pylori infection rate compared to the solitary cancer cohort [5]. Independent risk factors for SMEGC include advanced age, male, atrophic gastritis, and moderate to severe intestinal epithelial metaplasia of the gastric mucosa [6]. Nakajima et al. conducted a study that demonstrated the influence of physicochemical factors on methylation in gastric mucosa [23]. They found that the methylation level in the gastric mucosa was higher in multiple gastric cancers than in single cancers. These results suggest differences in the degree of mucosal atrophy and intestinal metaplasia between patients with SMGN and those with single carcinomas. Takaoka et al. identified that methylation of the mutL homolog 1 promoter plays a significant role in the development of the disease. Inactivation of the mismatch repair system leads to the accumulation of somatic mutations, contributing to the progression of SMEGC [7]. Therefore, the presence and severity of these mucosal changes should be carefully evaluated during the diagnosis and treatment of SMGN to assess the risk of developing gastric cancer [20].
This study has several limitations. First, although efforts were made to expand the sample size, there were still relatively few cases of undifferentiated carcinoma and submucosal infiltration in SMGN, limiting the comprehensive exploration of these cases. Further expanded samples are warranted in future studies to provide more information on these specific SMGN subtypes. Second, some patients were referred during the review process, leading to the loss of post-ESD gastroscopy review data for these individuals. This may have resulted in an underestimation of the incidence of SMGNs, as some cases may have been missed or not included in the analysis. These limitations highlight the need for larger sample sizes and careful data collection to enhance the robustness and generalizability of the findings in future studies investigating SMGN.
Conclusions
Through the study of patients with SMGN who underwent ESD, we found that the correlation coefficients for microscopic features were higher than those for gastroscopy. Our study findings suggest a significant positive correlation between the main and minor lesions in terms of pathological stage and depth of infiltration, with a similar spatial distribution of the lesions and the gastroscopic morphology. There was a statistically significant difference between the size of the main and minor lesions and a positive correlation.
References
Jeong SH, An J, Kwon KA, Lee WK, Kim KO, Chung J-W, Kim YJ, Park DK, Kim JH (2017) Predictive risk factors associated with synchronous multiple early gastric cancer. Medicine (Baltimore) 96:e7088. https://doi.org/10.1097/MD.0000000000007088
Cho J-H, Cha S-W, Kim HG, Lee TH, Cho JY, Ko WJ, Jin S-Y, Park S (2016) Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc 30:3762–3773. https://doi.org/10.1007/s00464-015-4672-1
Kasuga A, Yamamoto Y, Fujisaki J, Okada K, Omae M, Ishiyama A, Hirasawa T, Chino A, Tsuchida T, Hoshino E, Igarashi M (2013) Simultaneous endoscopic submucosal dissection for synchronous double early gastric cancer. Gastric Cancer 16:555–562. https://doi.org/10.1007/s10120-012-0218-6
Wan J, Fang Y, Jiang H, Wang B, Xu L, Hu C, Chen H, Ding X (2023) Endoscopic screening for missed lesions of synchronous multiple early gastric cancer during endoscopic submucosal dissection. Gastroent Res Pract 2023:1–8. https://doi.org/10.1155/2023/2824573
Xu SS, Chai NL, Tang XW, Linghu EQ, Wang SS, Feng XX, Li B (2021) A predictive risk-scoring model for multiple synchronous early gastric cancers or gastric dysplasia before initial endoscopic resection. J Dig Dis 22:637–644. https://doi.org/10.1111/1751-2980.13050
Zhao B, Mei D, Luo R, Lu H, Bao S, Xu H, Huang B (2020) Clinicopathological features, risk of lymph node metastasis and survival outcome of synchronous multiple early gastric cancer. Clin Res Hepatol Gastroenterol 44:939–946. https://doi.org/10.1016/j.clinre.2020.02.004
Takaoka S, Hirotsu Y, Ohyama H, Mochizuki H, Amemiya K, Oyama T, Ashizawa H, Yoshimura D, Nakagomi K, Hosoda K, Suzuki Y, Kojima Y, Omata M (2019) Molecular subtype switching in early-stage gastric cancers with multiple occurrences. J Gastroenterol 54:674–686. https://doi.org/10.1007/s00535-019-01547-z
Lee HJ, Lee YJ, Lee JY, Kim ES, Chung WJ, Jang BK, Park KS, Hwang JS, Cho KB (2018) Characteristics of synchronous and metachronous multiple gastric tumors after endoscopic submucosal dissection of early gastric neoplasm. Clin Endosc 51:266–273. https://doi.org/10.5946/ce.2017.109
Jang MY, Cho JW, Oh WG, Ko SJ, Han SH, Baek HK, Lee YJ, Kim JW, Jung GM, Cho YK (2013) Clinicopathological characteristics of synchronous and metachronous gastric neoplasms after endoscopic submucosal dissection. Korean J Intern Med 28:687. https://doi.org/10.3904/kjim.2013.28.6.687
Kim JH, Jeong SH, Yeo J, Lee WK, Chung DH, Kim KO, Chung J-W, Kim YJ, Kwon KA, Park DK (2016) Clinicopathologic similarities of the main and minor lesions of synchronous multiple early gastric cancer. J Korean Med Sci 31:873. https://doi.org/10.3346/jkms.2016.31.6.873
Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K (2021) Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc 33:4–20. https://doi.org/10.1111/den.13883
Moertel CG, Bargen JA, Soule EH (1957) Multiple gastric cancers; review of the literature and study of 42 cases. Gastroenterology 32:1095–1103
Participants In The Paris Workshop (2003) The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc 58:S3–S43. https://doi.org/10.1016/S0016-5107(03)02159-X
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA, the WHO Classification of Tumours Editorial Board (2020) The 2019 WHO classification of tumours of the digestive system. Histopathology 76:182–188. https://doi.org/10.1111/his.13975
Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, Lim SG, Hwang JC, Cheong JY, Yoo BM, Lee KJ, Kim JH, Cho SW (2013) How can we predict the presence of missed synchronous lesions after endoscopic submucosal dissection for early gastric cancers or gastric adenomas? J Clin Gastroenterol 47:e17–e22. https://doi.org/10.1097/MCG.0b013e31825c0b69
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123. https://doi.org/10.1007/s10120-011-0042-4
Yasuda M, Kuwano H, Watanabe M, Toh Y, Ohno S, Sugimachi K (2000) p53 expression in squamous dysplasia associated with carcinoma of the oesophagus: evidence for field carcinogenesis. Br J Cancer 83:1033–1038. https://doi.org/10.1054/bjoc.2000.1443
Lee HL, Eun CS, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS, Koh DH (2010) When do we miss synchronous gastric neoplasms with endoscopy? Gastrointest Endosc 71:1159–1165. https://doi.org/10.1016/j.gie.2010.01.011
Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, Nishihara A, Nakanishi F, Nakahara M, Ogiyama H, Kinoshita K, Yamada T, Iijima H, Tsujii M, Takehara T (2013) Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 62:1425–1432. https://doi.org/10.1136/gutjnl-2011-301647
Takeuchi D, Koide N, Suzuki A, Shimizu F, Koyama Y, Ehara T, Yamamoto Y, Koyama M, Nakamura S, Kitazawa M, Miyagawa Y, Miyagawa S (2018) High incidence of other primary malignancies in patients with synchronous multiple gastric cancers “a multi-center retrospective cohort study.” Oncotarget 9:20605–20616. https://doi.org/10.18632/oncotarget.25027
Wittekind C, Klimpfinger M, Hermanek P, Tannapfel A (1997) Multiple simultaneous gastric carcinomas. Br J Cancer 76:1604–1609. https://doi.org/10.1038/bjc.1997.604
Seo JH, Park JC, Kim YJ, Shin SK, Lee YC, Lee SK (2010) Undifferentiated histology after endoscopic resection may predict synchronous and metachronous occurrence of early gastric cancer. Digestion 81:35–42. https://doi.org/10.1159/000235921
Nakajima T, Maekita T, Oda I, Gotoda T, Yamamoto S, Umemura S, Ichinose M, Sugimura T, Ushijima T, Saito D (2006) Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol Biomark Prev 15:2317–2321. https://doi.org/10.1158/1055-9965.EPI-06-0436
Acknowledgements
None.
Funding
This work was supported by the Natural Science Foundation of Fujian Province [Grant Number 2021J01388], and the High-level Hospital Foster Grants from Fujian Provincial Hospital [Grant Number 2019HSJJ22]. The sponsors had no role in study design, data collection, analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Yudai Chen, Chaoying Fang, Jianmin Huang, Hui Pan, Liping He, Chenlin Zhuang, and Xiaoling Zheng have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Fang, C., Huang, J. et al. The correlation between the main and minor lesions of synchronous multiple gastric neoplasms assessed gastroscopically and microscopically. Surg Endosc 38, 1211–1221 (2024). https://doi.org/10.1007/s00464-023-10624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10624-7