Abstract
Background
Submucosal tunneling techniques have expanded the horizon of therapeutic endoscopy. One such procedure, submucosal tunneling endoscopic resection (STER), enables the endoscopic removal of gastrointestinal (GI) sub-epithelial tumors. In this study, we aimed to evaluate the safety and efficacy of STER in patients with sub-epithelial lesions localized to the upper GI tract.
Methods
Consecutive subjects with a sub-epithelial lesion of ≥ 1 cm size in the upper GI tract were enrolled in the study. STER was performed using the standard technique in an endoscopy suite. A modified technique (double-opening STER) was used in cases with difficult en bloc resection of the tumor. Outcome measures included technical success, en bloc resection rates, adverse events, and recurrence.
Results
A total of 104 patients with sub-epithelial tumors were evaluated for STER. Of them, 44 subjects (mean age 44.68 ± 12.82, 52.3% males) underwent standard STER. Majority (31, 70.4%) of the lesions were located in the esophagus and cardia. Technical success and en bloc removal of the tumor were achieved in 97.7% and 88.4% of cases, respectively. There was no major adverse event. Minor adverse events were recorded in 7 (15.9%) cases. Majority (31, 70.4%) of the tumors originated from muscularis propria, followed by submucosa (8, 18.2%) and muscularis mucosa (5, 11.4%). The most common histological diagnosis was leiomyoma (59.1%) followed by GI stromal tumors (20.4%). At a mean follow up of 12.36 ± 7.63 months, there was no incidence of tumor recurrence in en bloc as well as piecemeal resection groups.
Conclusion
STER is a safe and efficacious procedure for sub-epithelial tumors in the upper GI tract. Novel strategies need to be developed to ensure en bloc removal of large lesions.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sub-epithelial tumors of the gastrointestinal (GI) tract are protruding lesions covered by normal-appearing mucosa [1]. The current management protocol for these lesions is unclear due to limited data on their natural history and behavior in long-term. Majority of these lesions are benign and asymptomatic. Therefore, endoscopic surveillance at regular intervals is one of the options for benign-appearing sub-epithelial lesions. However, the optimum surveillance interval is not clear and some of the larger lesions may harbor malignant potential.
The management options in these cases include endoscopic resection or surgery (thoracoscopic enucleation or laparoscopic). Surgery is often not acceptable to the patients as well as the treating physicians due to its invasive nature and associated morbidities. With recent innovations in therapeutic endoscopy, a large proportion of these lesions can be removed with minimally invasive techniques [2]. The major endoscopic techniques for removal of sub-epithelial tumors include submucosal tunneling endoscopic resection (STER), endoscopic submucosal dissection, endoscopic submucosal excavation, and endoscopic full-thickness resection (EFTR) [3].
In this study, we aimed to analyze the safety and efficacy of STER for sub-epithelial lesions in the upper GI tract.
Methods
Consecutive patients with a diagnosis of sub-epithelial lesion in the esophagogastric tract were enrolled in the study from January 2016 to October 2018. Inclusion criteria were lesion ≥ 1 cm in size, age > 18 years, absence of invasion beyond the GI tract, and willing for informed consent. Exclusion criteria included lesions with high-risk features of malignancy on imaging, tumors with surface ulceration, and predominant exophytic component (extraluminal >intraluminal), contraindications to general anesthesia, coagulopathy (international normalized ratio > 1.5; platelets < 50,000), presence of portal hypertension, pregnancy, current use of antiplatelets or anticoagulants, and failure to provide consent. High-risk features for malignancy on endosonography and/or computed tomography included breach of serosal layer, anechoic areas within the tumors, and adjacent malignant-appearing lymph nodes. These features were mainly utilized for suspected GI stromal tumors and neuroendocrine tumors.
Informed consent was obtained from the eligible participants. The study was approved by ethics review committee and institutional review board.
Preoperative evaluation
All the patients underwent preoperative imaging including endoscopic ultrasound and contrast computed tomography (CT) to determine the size, vascularity, relationship with adjacent structures, and the layer of origin. Coagulation parameters were checked in all the patients prior to proceeding for resection.
STER technique
All the STER procedures were performed by three experienced endoscopists (ZN, MR, DNR) in an endoscopy suit under general anesthesia. Tracheal intubation was done using a cuffed flexo-metallic endotracheal tube after induction with intravenous propofol and atracurium besylate in all the cases. We have previously published the details of mechanical ventilation for per-oral endoscopic myotomy in patients with achalasia [4].
The patients were kept in supine position for esophageal tumors and left lateral position for gastric lesions. An intravenous broad-spectrum antibiotic was administered preoperatively about half hour prior to commencement of the procedure.
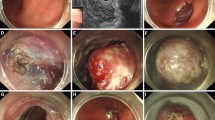
The steps of the STER procedure are as follows: (1) A submucosal-lifting injection consisting of diluted indigo carmine solution was given 2–4 cm proximal to the site of the lesion (Fig. 1a, b); (2) mucosal incision of about 2 cm in size was made using the new triangular tip knife (Fig. 1c); (3) submucosal fibers were cleared along the edges and apex of the incision and scope introduced inside the tunnel; (4) submucosal tunnel was created using the same knife and extended until about 1–2 cm beyond the sub-epithelial tumor (Fig. 1d); (5) tumor was dissected from the surrounding attachments and retrieved from the tunnel using a polypectomy snare (Fig. 1e, f); (6) closure of the mucosal incision was done using endoclips (Fig. 1g–i).
Submucosal tunneling endoscopic resection in a case of gastrointestinal GI stromal tumor. a Bulge in the antrum representing GI stromal tumor (short arrows pointing). b Submucosal injection of saline mixed with indigo carmine proximal to the lesion (single arrow pointing towards the bulge). c Mucosal incision of about 2 cm size proximal to the lesion (three short arrows pointing towards the length of incision). d Submucosal tunneling using triangular knife (single arrow pointing towards the knife and two arrows pointing towards the blue-stained submucosal fibers). e Dissection of the tumor from surrounding attachments (two arrows pointing towards the submucosal lesion attached to the surrounding tissue). f Removal of the tumor using a snare (arrow pointing towards the braided snare). g Wide-gaping mucosal incision visualized after removal of the tumor. h Closure of the incision using endoloop-clip method (arrows pointing towards the blue colored endoloop). i Endoscopic view after completion of mucosal incision closure
The technique of STER was modified in some cases with tumor originating from muscularis mucosa. After creating a submucosal pocket, the tumor was removed intact along with the overlying mucosa. The mucosal defect was subsequently closed with endoclips.
In cases where the retrieval of tumor was difficult due to large size, a double-opening technique was utilized (DO-STER). In this technique, the initial steps are similar to the standard technique (Fig. 2a–c). After dissecting the tumor from its surrounding attachments, a small mucosal opening was created at the distal end of the submucosal tunnel (Fig. 2d, e). Subsequently, the tumor was pushed out of the opening into GI lumen and retrieved using a snare (Fig. 2f). Finally, the mucosal opening and the initial mucosal incision were closed with endoclips (Fig. 2g, h).
Modified submucosal tunneling endoscopic resection technique in a case with large esophageal leiomyoma. a Endoscopic view of a large esophageal leiomyoma (black arrows pointing towards the tumor). b Submucosal injection using diluted indigo carmine solution followed by mucosal incision (black arrows pointing towards the length of the incision). c Submucosal tunneling (note the coagulation of blood vessel [thick black arrow] using coagulation forceps [thin black arrow]). d Dissection of submucosal tumor (short black arrow) from the surroundings (long black arrow represents the surrounding attachments). e Creation of a second mucosal opening (two thin white arrows) at distal margin of tumor (thick white arrow). f Extraction of tumor (thick white arrow) from gastric lumen using a snare (thin white arrow). g Closure of the distal mucosal opening using endoclips. h Closure of initial mucosal incision using endoclips
All the retrieved samples were sent for histopathological evaluation. Immunohistochemistry (IHC) was performed for detailed assessment and classification of the sub-epithelial tumors. The following IHC markers were used: smooth muscle actin, desmin (leiomyoma); CD117 (gastrointestinal stromal tumors); chromogranin A, synaptophysin (neuroendocrine tumors); CD68, S100 (granular cell tumors); and thyroid transcription factor 1 (gastric duplication cyst).
Post-procedure protocol
After the procedure, the patients were monitored in the intensive care unit for 6–8 h. Oral liquids were allowed the next day and a soft-pureed diet was initiated on third postoperative day until the end of first week.
The patients were followed up at 1 month, 3 months, and 6 months after the procedure. An upper GI endoscopy was performed at 1 and 6 months after the procedure.
Outcome measures
The following parameters were recorded: technical success in completing the procedure, procedure duration, intraoperative adverse events, en bloc resection rate, and incidence of recurrence at 6 months.
Definitions
Technical success
Successful removal of the entire tumor with the tunneling technique
Procedure duration
Time taken from the submucosal-lifting injection to the closure of incision with endoclips
Adverse events
Intraoperative events requiring an active intervention like drainage of capno-thorax or capno-peritoneum, closure of mucosal perforations or events requiring premature cessation of the procedure, and major bleeding necessitating blood transfusion were considered as adverse events. Minor intra-procedural bleeding episodes and incidentally detected insufflation-related events not requiring any intervention were not considered as adverse events.
Recurrence
Any visible protrusions, except for the clip-related mucosal hypertrophy, at the site of previous tumors confirmed using endoscopic ultrasonography were defined as recurrences.
Devices and accessories [5]
The following equipment were used for the STER procedure: endoscope (Olympus GIF HQ 190; Olympus Corp., Tokyo, Japan); tapered tip transparent cap (DH-28GR; Fujifilm, Tokyo, Japan); carbon dioxide insufflator (UCR; Olympus Corp., Tokyo, Japan) with an extra-low-flow gas tube (MAJ-1816; Olympus Corp., Tokyo, Japan); electrosurgical unit (VIO300D; ERBE, Tübingen, Germany); insulated-tip knife (KD-611L; Olympus Corp., Tokyo, Japan); triangle-tip knife with integrated waterjet (TriangleTipKnife J, KD-645L; Olympus Corp., Tokyo, Japan); coagulation forceps (Coagrasper G, FD-412LR; Olympus Corp., Tokyo, Japan); endoclips (EZClip, HX-610–090L; Olympus Corp., Tokyo, Japan).
The settings on electrosurgical unit were ENDO CUT Q at 50 W, effect 2 for submucosal tunneling using spray coagulation mode, and 80 W, effect 5 in soft coagulation mode.
Statistics
The data are presented as mean ± standard deviation. Student’s t test was used for continuous variables and Chi-squared test for categorical variables. A p-value < 0.05 was considered statistically significant.
Results
A total of 104 patients were diagnosed as upper GI sub-epithelial tumors during the study period and were further evaluated for STER. Of them, 37 patients were ineligible for various reasons and 23 did not agree for the procedure. Ten (9.6%) sub-epithelial tumors were located in fundus (7) or lower down along the lesser curvature (3). STER was not performed for these lesions due to difficult location and angulation of the endoscope.
Overall, 44 patients (mean age 44.68 ± 12.82 years, 52.3% males) underwent STER procedure and were included in the final analysis (Fig. 3).
Thirty-one (70.45%) tumors were located in the esophagus and cardia of the stomach. The layer of origin of sub-epithelial tumors was muscularis mucosa in 5 (11.36%), submucosa in 8 (18.18%), and muscularis propria in 31 (70.45%) patients. The mean size of sub-epithelial lesions was 2.54 ± 1.16 cm (range 1–6 cm) (Table 1).
Outcomes
STER procedure was successfully completed in 43 (97.7%) patients. In one patient, the tumor located in gastric cardia could not be removed due to predominantly exophytic component. The mean procedure time was 51.05 ± 17.87 min. Procedure duration was significantly higher in sub-epithelial lesions > 4 cm in size as compared with that in smaller lesions (86.75 ± 14.22 vs. 57.14 ± 10.50 vs. 36.79 ± 8.10 min; p = 0.0001). Majority (39, 90.70%) of the lesions were resected by the standard technique of STER. In 4 cases, a modified technique of STER (double-opening STER) was utilized to remove the tumors.
En bloc resection of the tumor was achieved in 38 (88.37%) patients who underwent successful STER. The mean size of tumor was significantly greater in patients with piecemeal resection (n = 5) as compared with that in the en bloc group (4.60 ± 0.85 vs. 2.30 ± 1.05 cm). The histological diagnosis in majority of the resected lesions was leiomyoma (60.46%) in the esophagus, and GI stromal tumor (20.93%) in the stomach. At a mean follow up of 12.36 ± 7.63 months, there was no recurrence in either of the groups, i.e. en bloc resection or piecemeal resection group (Tables 2 and 3).
Adverse events
There was no major adverse event. Insufflation-related events were recorded in 8 (18.18%) patients including capno-thorax (1), capno-peritoneum (5), and retro-peritoneal CO2 (2). Of them, an intervention was required in 5 (11.36%) patients. All the patients with capno-peritoneum were managed with needle decompression using 18G intravenous cannula. The procedure was temporarily withheld in cases of capno-thorax and accumulation of retro-peritoneal CO2. Mucosal injury requiring closure occurred in 2 patients (4.54%). Overall, an adverse event defined by the requirement of an intervention occurred in 7 (15.91%) patients. Mean procedure time was significantly higher in those with adverse events (66.90 ± 21.09 vs. 46.38 ± 14.01 min; p < 0.05) (Table 2).
Discussion
In this study, we found that STER is a safe and efficacious procedure for subepithelial tumors located in the upper GI tract. In addition, the procedure can be safely accomplished in an endoscopy suit.
Subepithelial tumors are not uncommon in the upper GI tract. Vast majority of these lesions are detected incidentally on gastroscopy performed for other reasons. There is no well-established guideline for the management of these tumors. Endoscopic surveillance is an option given the benign nature of disease in most of the cases. However, there is no standard protocol for surveillance. Moreover, lifelong surveillance adds financial and psychological burden to the patients. Recent innovations in third-space endoscopy have expanded the therapeutic armamentarium for the management of many GI disorders including subepithelial tumors [2].
In this study, we analyzed the safety and efficacy of STER for the resection of upper GI sub-epithelial tumors. We included patients with upper GI sub-epithelial tumors larger than 1 cm in size. Smaller sub-epithelial lesions (< 1 cm) rarely harbor malignant potential and therefore were not considered for resection. All the patients were counseled, and eligible cases provided with the options of endoscopic surveillance, endoscopic resection, and surgery. Nearly two-thirds of the eligible patients agreed for endoscopic resection of the lesions. This implies that when given a minimally invasive option, patients prefer endoscopic resection over surveillance.
We could successfully remove most of the sub-epithelial lesions using STER technique. STER was unsuccessful in one lesion probably due to large size (3.5 cm), predominantly exophytic location, and origin from deep muscularis propria layer. This signifies the importance of imaging and proper selection of cases before proceeding to endoscopic resection with tunneling technique [6]. Other lesions not appropriate for STER include those located in the gastric fundus and lower part of lesser curvature due to retroflexed position of endoscope [7].
In the current study, en bloc resection could be achieved in majority (88%) of the cases. However, piecemeal resection had to be performed in five patients with large tumors (mean size > 4 cm). The results of our study are in concordance with a recent systematic review in which the pooled rate of en bloc resection was 94.6% (95% CI 91.5–96.7%) [8]. Similarly, large size (> 20 mm) and irregular shape have been found to be the predictors for failure of en bloc resection in previous studies [9, 10].
In this study, we also evaluated the safety and efficacy of a modified technique of STER, i.e. submucosal tunneling endoscopic resection with double opening (DO-STER) [11]. The main objective of the modified technique was to ensure en bloc removal of larger sub-epithelial tumors. In this technique, a second mucosal opening was created at the lower end of tunnel which in turn allowed the tumor to be pushed away into the esophageal/gastric lumen. Subsequent dissection of the tumor from the surrounding tissue was performed easily using the standard technique. We found this technique to be especially useful in en bloc resection of relatively large sub-epithelial tumors.
There was no major complication and all the minor adverse events (16%) could be successfully managed intra-operatively. Similar rate of adverse events has been reported in previous studies [12,13,14]. In a large study, the overall incidence of complications was 23.4%. However, only 10.0% of the complications required an intervention [14]. These included mucosal injury (1%), major bleeding (1.7%), major pneumothorax (3.1%), and thoracic effusion (3.8%). This implies that STER can be safely applied for the management of subepithelial tumors localized to the upper GI tract. Majority of the adverse events during third-space endoscopy procedures are insufflation-related and do not require an active intervention [2]. In this study, we predefined adverse events and did not include inconsequential events like subcutaneous emphysema, superficial mucosal injuries, and incidentally detected insufflation-related events like small capno-peritoneum not requiring drainage [15].
Besides STER, other non-tunneling endoscopic techniques for resection of subepithelial lesions include endoscopic submucosal evacuation, EFTR, and endoscopic submucosal dissection [3]. However, STER is preferable in upper GI subepithelial lesions for the following reasons. First, endoscopic submucosal dissection (ESD) is not feasible for tumors arising from muscularis propria and, therefore, cannot be utilized in a sizeable proportion of these patients. Second, the chances of leak and insufflation-related events are theoretically less with STER as compared with EFTR due to the preservation of the mucosal flap in the former [12]. Finally, the closure is more difficult after EFTR due to wider gaping of the wound. In a comparative study, patients who received EFTR for gastric GI stromal tumors had a longer suture time and needed more clips to close the gastric-wall defect [16].
There are several strengths of our study. This is the first study from India establishing the safety and efficacy of STER for upper GI sub-epithelial tumors. The data was extracted from a prospectively maintained database with standardized reporting of adverse events.
However, certain drawbacks are noteworthy including a small sample size and lack of a comparison arm like EFTR or endoscopic submucosal excavation. In addition, endosonography or cross-sectional imaging to exclude recurrence was performed in a minority of patients.
STER is a safe and effective procedure for the resection of sub-epithelial lesions in the upper GI tract. The procedure can be safely performed in an endoscopy suit. Further refinements in the technique, devices, and accessories are required to improve the en bloc resection rates.
References
Zhang J, Huang K, Ding S, et al. Clinical applicability of various treatment approaches for upper gastrointestinal submucosal tumors. Gastroenterol Res Pract. 2016;2016: https://doi.org/10.1155/2016/9430652
Nabi Z, Nageshwar Reddy D, Ramchandani M. Recent advances in third-space endoscopy. Gastroenterol Hepatol (NY). 2018;14:224–32.
Du C, Linghu E. Submucosal tunneling endoscopic resection for the treatment of gastrointestinal submucosal tumors originating from the muscularis propria layer. J Gastrointest Surg. 2017;21:2100–9.
Darisetty S, Nabi Z, Ramchandani M, Chavan R, Kotla R, Nageshwar Reddy D. Anesthesia in per-oral endoscopic myotomy: a large tertiary care centre experience. Indian J Gastroenterol. 2017;36:305–12.
Nabi Z, Ramchandani M, Chavan R, et al. Peroral endoscopic myotomy in treatment-naive achalasia patients versus prior treatment failure cases. Endoscopy. 2018;50:358–70.
Chu Y, Qiao X, Gao X, et al. Combined EUS and CT for evaluating gastrointestinal submucosal tumors before endoscopic resection. Eur J Gastroenterol Hepatol. 2014;26:933–6.
Lu J, Lu X, Jiao T, Zheng M. Endoscopic management of upper gastrointestinal submucosal tumors arising from muscularis propria. J Clin Gastroenterol. 2014;48:667–73.
Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017;31:49–63.
Li Z, Gao Y, Chai N, et al. Effect of submucosal tunneling endoscopic resection for submucosal tumors at esophagogastric junction and risk factors for failure of en bloc resection. Surg Endosc. 2018;32:1326–35.
Chen T, Zhou PH, Chu Y, et al. Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg. 2017;265:363–9.
Zhang Q, Cai JQ, Xiang L, Wang Z, de Liu S, Bai Y. Modified submucosal tunneling endoscopic resection for submucosal tumors in the esophagus and gastric fundus near the cardia. Endoscopy. 2017;49:784–91.
Lu J, Jiao T, Zheng M, Lu X. Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc. 2014;28:3401–7.
Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28:524–30.
Chen T, Zhang C, Yao LQ, et al. Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy. 2016;48:149–55.
Nabi Z, Reddy DN, Ramchandani M. Adverse events during and after per-oral endoscopic myotomy: prevention, diagnosis, and management. Gastrointest Endosc. 2018;87:4–17.
Tan Y, Tang X, Guo T, et al. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc. 2017;31:3376–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ZN, MR, MS, SD, RK, GVR, and DNR declare that they have no conflict of interest.
Ethics statement
The study was performed conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nabi, Z., Ramchandani, M., Sayyed, M. et al. Outcomes of submucosal tunneling endoscopic resection in upper gastrointestinal sub-epithelial tumors. Indian J Gastroenterol 38, 509–517 (2019). https://doi.org/10.1007/s12664-019-00988-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-019-00988-x