Abstract
Background
Since its widespread acceptance for the treatment of early gastric cancer, laparoscopic gastrectomy has been gaining popularity as a treatment option for advanced gastric cancer. However, laparoscopic total gastrectomy (LTG) with splenectomy is seldom performed, because of its difficulty of removal of station 10 lymph nodes; splenectomy is technically essential for complete removal of these lymph nodes. The purpose of this study was to describe the details of the LTG procedure and to evaluate the short- and long-term outcomes of LTG with splenectomy.
Methods
Of 725 consecutive patients with gastric cancer who underwent laparoscopic gastrectomy with lymph node dissection in our institution from January 1996 to December 2012, 18 consecutive patients who underwent LTG with splenectomy were enrolled in this study.
Results
No operative mortality occurred, and the pathological margins were free from cancer cells in all patients. The mean operation time was 388 min (range 324–566 min). The mean volume of blood loss was 45 ml (range 5–347 ml), and the mean number of dissected lymph nodes was 51 (range 40–105). Postoperative morbidity occurred in six patients (33.3 %) (each with grade B postoperative pancreatic fistula, postoperative bleeding, chylous ascites, atelectasis, ileus, and intra-abdominal infection). Five patients (27.8 %) developed recurrence (four in the peritoneum and one in the liver), and the overall 3- and 5-year survival rates were 83.0 and 72.6 %, respectively.
Conclusions
Considering the 0 % mortality rate and low rates of postoperative morbidity and locoregional recurrence, LTG with splenectomy is technically and oncologically acceptable. This procedure can be expanded to include advanced gastric cancer, which generally requires splenectomy for lymph node dissection.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Laparoscopic techniques are widely performed as minimally invasive procedures for various cancers. Since Kitano et al. reported laparoscopy-assisted distal gastrectomy in 1991 [1], the number of laparoscopic gastrectomy procedures has been increasing for a number of reasons, including low levels of pain and blood loss, early resumption of bowel movements, and short hospital stays [2–4].
Improvements in laparoscopic techniques and surgical instruments have raised interest in expanded adaptation of laparoscopic surgery to advanced gastric cancer. Although laparoscopic distal gastrectomy with radical lymph node dissection has been safely performed in several institutions [5–9], few reports have described laparoscopic total gastrectomy (LTG) with radical lymph node dissection [10, 11]. The reasons for the low popularity of LTG with radical lymph node dissection is the difficulty of removal of station 10 lymph nodes; splenectomy is technically essential for complete removal of these lymph nodes. And simultaneous splenectomy may cause the severe complications after operation.
Although splenectomy for lymph node dissection to improve survival of patients with gastric cancer is controversial, the Japanese Gastric Cancer Association guideline states that splenectomy is recommended for complete removal of the station 10 lymph nodes in patients with advanced gastric cancer [12]. However, no reports have focused on the technical and oncological feasibility of LTG with splenectomy for gastric cancer.
The purpose of this study was to describe the details of the LTG with radical lymphadenectomy procedure and evaluate the technical feasibility, safety, and short- and long-term outcomes of LTG with splenectomy for gastric cancer.
Patients and methods
In total, 725 patients with gastric cancer underwent laparoscopic gastrectomy with lymph node dissection at the Department of Surgery and Oncology, Kyushu University Hospital (Fukuoka, Japan) from January 1996 to December 2012. Since 2002, we have performed LTG with lymph node dissection in 149 patients. Of these 149 patients who underwent LTG, 18 underwent LTG with splenectomy for D2 lymph node dissection, and we enrolled these patients in this study.
Tumor/node/metastasis staging was based on the International Union Against Gastric Cancer Staging, 7th edition. We excluded from this study patients who underwent total remnant gastrectomy or simultaneous resection of other organs with the exception of the gallbladder. Individual data such as the clinical course and pathological findings were obtained from the medical records.
Surgical LTG procedure
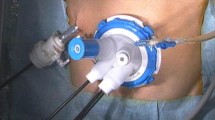
The operator stood on the right side of the patient. A Hasson trocar was inserted umbilically by the open technique for a 30° camera followed by insertion of four trocars (Fig. 1A). After dividing the duodenum, we performed nodal dissection along the celiac, common hepatic, proper hepatic, and proximal splenic arteries. The esophagus was then divided using a linear stapler. We obtained a wide view of the superior distal pancreatic area and hilum of the spleen, then began nodal dissection along the distal splenic artery (Fig. 1B). First, the posterior wall of the omental bursa was dissected along the superior pancreatic edge from the pancreatic body to tail. We exposed the splenic artery and vein while preserving the great and caudal pancreatic vessels to completely dissect the station 11 lymph nodes. We then performed a splenectomy for complete dissection of the station 10 lymph nodes. Before ligation of the splenic vessels at the hilum of the spleen, we applied a curved vessel clamp to the splenic artery. Each branch to the spleen was then ligated and cut individually. We used 4-0 Prolene (Ethicon Inc., Somerville, NJ, USA) to ligate the artery. The Prolene was lifted to obtain a good operative view and dissect the lymph nodes behind the pancreatic tail (Fig. 1C). We usually preserved the caudal pancreatic arteries and veins (Fig. 1D, E). Finally, the adipose tissue located on the posterior side of the pancreatic tail was dissected along the parenchyma to complete the lymph node dissection (Fig. 1D–G). After removing the vessel clamp, the specimen was retrieved thorough the elongated 5-cm-long umbilical incision. We routinely placed two closed drains in the left subphrenic space, behind the esophagojejunostomy site.
A Placement of surgical ports. A 12-mm Hasson trocar was placed under the umbilicus and extended upward for a 50-mm minilaparotomy. B Laparoscopic view during dissection along the proximal side of the splenic artery. C The superior branch of the splenic artery (superior polar artery) was identified and cut using 4-0 prolene to ligate the artery. D and E Laparoscopic view during the dissection of the lymph nodes along the distal side of the splenic artery. The caudal pancreatic arteries were preserved. F and G Laparoscopic view after dissection of the lymph nodes, proximal side (F) and distal side (G). H Schema of the arteries around the pancreas. SPA splenic artery, SPV splenic vein, PCPA proximal caudal pancreatic artery, DCPA distal caudal pancreatic artery, STA superior terminal artery, ITA inferior terminal artery, IPA inferior polar artery, LN lymph nodes
Postoperative course
Postoperative complications were graded according to the Clavien–Dindo classification [13]. A postoperative pancreatic fistula (POPF) was defined as a high-level drainage output of any measurable volume of fluid on or after postoperative day (POD) 3 with an amylase content of >3 times that in the serum [14]. We measured the level of amylase in the drainage fluid (d-amy) on POD 1 and 3. In patients with more than one drain, the highest d-amy level was taken as the representative value. If the d-amy level was high, we continued to measure it after POD 3. We removed the drain before POD 3, when the d-amy level was low. In such cases, POPF was suspected on the basis of physical and biochemical findings, such as abdominal pain, an elevated temperature (>38 °C), a serum leukocyte count of >10,000 cells/mm3, an increased C-reactive protein level, and the presence of peripancreatic fluid detected by computed tomography.
Results
Clinicopathologic patient characteristics
The clinical characteristics of the 18 patients are shown in Table 1. The patients comprised 11 (61.1 %) males and 7 (38.9 %) females. The median patient age was 61 years (range 35–74 years). The median body mass index was 21.5 kg/m2 (range 15.1–28.9 kg/m2). Two patients had a clinical diagnosis of submucosal invasion, and these two patients had clinically positive lymph nodes. The laparoscopic procedure was not converted to the conventional open method in any patient.
Postoperative outcome
There was no operative mortality, and the pathological margin was free from cancer cells in all patients. The mean operation time was 388 min (range 324–566 min). The mean volume of blood loss was 45 ml (range 5–347 ml). The mean number of dissected lymph nodes was 51 (range 41–105). The mean postoperative duration of hospitalization was 12 days (range 8–25 days) (Table 2). Six patients (33.3 %) developed postoperative complications (one each with atelectasis, ileus, grade B POPF, intra-abdominal infection, chylous ascites, and intra-abdominal infection) (Table 3). The patient who developed postoperative bleeding underwent a reoperation to achieve hemostasis. We monitored the drain and serum amylase levels after the operation. The mean d-amy level on POD 1 and 3 were 2,499 and 642 IU/l, respectively (Table 4). Of the 18 patients, only one was diagnosed with grade B POPF on POD 7 and successfully treated with antibiotics and drainage. No patients developed a grade C POPF (Table 4). The median follow-up time was 54.7 months. Five (27.8 %) patients developed tumor recurrence [4 (22.2 %) peritoneal recurrence, 1 (5.6 %) liver recurrence]. There were no cases of locoregional or lymphatic recurrence. Two of the five patients with recurrent tumors had already been diagnosed with stage IV disease at the time of the operation based on positive peritoneal lavage cytology results. The overall 3- and 5-year survival rates were 83.0 and 72.6 %, respectively (Table 5).
Discussion
We herein report the technical feasibility, safety, and short- and long-term outcomes of LTG with splenectomy for patients with gastric cancer. Although the effect of splenectomy for lymph node dissection is still unclear, the Japanese Gastric Cancer Association recommends simultaneous splenectomy for selected patients with advanced gastric cancer. Therefore, we have been performing LTG with splenectomy for patients with advanced gastric cancer invading the greater curvature of the upper third of the stomach, pancreatic parenchyma, or spleen. Patients with positive lymph nodes along the distal splenic artery also underwent LTG with splenectomy.
In our institution, the application of laparoscopic surgery has been gradually expanded, and we now perform gastrectomy laparoscopically for almost all cases of both advanced and early gastric cancer. We consider it extremely important to ensure the same quality of open surgery in terms of necessary and sufficient nodal dissection and the safety and efficiency of reconstruction.
In this study, we did not compare LTG with open total gastrectomy (OTG) because of the different background of the study period; for example, the introduction of a critical pathway and the indications for neoadjuvant therapy are quite different between LTG and OTG in our department. Therefore, we evaluated recently published data regarding OTG with splenectomy and found a postoperative morbidity rate of 59.5 % [15]. Lee et al. [10] and Shinohara et al. [11] reported complication rates of LTG with D2 lymph node dissection of 42.6 and 33.0 %, respectively.
Considering that combined splenectomy has been reported to increase the postoperative complication rate [16], LTG with splenectomy in our institution seems to be technically safe and clinically feasible. In this study, there were no locoregional or lymphatic recurrences. Although four patients died of peritoneal recurrence after the operation, two of them already had stage IV disease at the time of gastrectomy and lived 27 and 57 months each. One of the other two patients with peritoneal recurrence showed subserosal invasion (pT3), and the other showed serosal invasion (pT4). We cannot completely deny the possibility of peritoneal seeding of cancer cells during the operation. However, Shinohara et al. [11] reported a peritoneal recurrence rate of 50 % in OTG, and we consider this outcome to be comparable from the viewpoint of oncological safety.
Only one patient had a grade B POPF, and this was the first patient to undergo LTG with splenectomy in our institution. Maruyama et al. [17] stated that a couple of caudal arteries were present at the end of the pancreas and that the blood supply to the pancreas would be preserved even when these arteries were ligated to allow for dissection of the lymph nodes along the splenic artery. However, we consider that the blood supply is not sufficient after ligating these arteries. Therefore, we usually preserve the great pancreatic artery and two caudal artery branches that we termed the proximal and distal caudal artery (Fig. 1D, E, H). Nobuoka et al. [18] also stated that preservation of the splenic artery, including the great pancreatic artery and caudal pancreatic arteries, is one of the most important factors to prevent POPF. The frequency of POPF greater than grade B was lower than that in OTG in previous studies, which reported the frequency of POPF greater than grade B after total gastrectomy with or without splenectomy to be 6.7–19.4 % [18–22]. Therefore, we consider that the frequency of POPF in our study is very low.
The mean d-amy levels on POD 1 and 3 were 2,499 and 642 IU/l, respectively. When compared with previously reported results, the d-amy levels in the LTG group seem to be comparable with those in the OTG group [21, 23]. The d-amy level is reportedly a useful predictor of POPF, and a d-amy level of >1,000 IU/l is a risk factor for pancreas-related complications [21, 23]. Therefore, the d-amy levels in the LTG group in this analysis were low, and LTG with splenectomy can be a safe method from the viewpoint of pancreatic complications after TG.
In conclusion, although the number of patients was limited and splenectomy for lymph node dissection to improve survival of patients with gastric cancer is controversial, we consider that LTG with splenectomy is technically and oncologically acceptable and can be expanded for advanced gastric cancer. Preservation of the caudal pancreatic vessels might reduce the incidence of POPF. The indication for LTG with splenectomy can be expanded for patients with gastric cancer, which requires complete station #10 lymph node dissection.
References
Kitano S, Iso Y, Moriyama M et al (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4(146–14):8
Shimizu S, Uchiyama A, Mizumoto K et al (2000) Laparoscopically assisted distal gastrectomy for early gastric cancer: is it superior to open surgery? Surg Endosc 14:27–31
Noshiro H, Shimizu S, Nagai E et al (2003) Laparoscopy-assisted distal gastrectomy for early gastric cancer: is it beneficial for patients of heavier weight? Ann Surg 238:680–685
Kitano S, Shiraishi N, Uyama I et al (2007) A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 245(68–7):2
Huscher CG, Mingoli A, Sgarzini G et al (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241(232–23):7
Hwang SI, Kim HO, Yoo CH et al (2009) Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc 23(1252–125):8
Satoh S, Okabe H, Kondo K et al (2009) Video. A novel laparoscopic approach for safe and simplified suprapancreatic lymph node dissection of gastric cancer. Surg Endosc 23:436–437
Tokunaga M, Hiki N, Fukunaga T et al (2009) Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection following standardization—a preliminary study. J Gastrointest Surg 13:1058–1063
Kanaya S, Haruta S, Kawamura Y et al (2011) Video: laparoscopy distinctive technique for suprapancreatic lymph node dissection: medial approach for laparoscopic gastric cancer surgery. Surg Endosc 25:3928–3929
Lee JH, Ahn SH, Park do J et al (2012) Laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer. World J Surg 36:2394–2399
Shinohara T, Kanaya S, Taniguchi K et al (2009) Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg 144:1138–1142
Japanese Gastric Cancer Association (2010) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011(14):113–123
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Bassi C, Dervenis C, Butturini G et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Goto H, Tokunaga M, Sugisawa N et al (2013) Value of splenectomy in patients with Siewert type II adenocarcinoma of the esophagogastric junction. Gastric Cancer 16(590–59):5
Sano T, Sasako M, Katai H et al (1997) Amylase concentration of drainage fluid after total gastrectomy. Br J Surg 84(1310–131):2
Maruyama K, Sasako M, Kinoshita T et al (1995) Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 19(532–53):6
Nobuoka D, Gotohda N, Konishi M et al (2008) Prevention of postoperative pancreatic fistula after total gastrectomy. World J Surg 32(2261–226):6
Kunisaki C, Makino H, Suwa H et al (2007) Impact of splenectomy in patients with gastric adenocarcinoma of the cardia. J Gastrointest Surg 11(1039–104):4
Otsuji E, Yamaguchi T, Sawai K et al (1996) End results of simultaneous splenectomy in patients undergoing total gastrectomy for gastric carcinoma. Surgery 120(40–4):4
Obama K, Okabe H, Hosogi H et al (2011) Feasibility of laparoscopic gastrectomy with radical lymph node dissection for gastric cancer: from a viewpoint of pancreas-related complications. Surgery 149(15–2):1
Sano T, Sasako M, Yamamoto S et al (2004) Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy—Japan Clinical Oncology Group study 9501. J Clin Oncol 22:2767–2773
Miki Y, Tokunaga M, Bando E et al (2011) Evaluation of postoperative pancreatic fistula after total gastrectomy with D2 lymphadenectomy by ISGPF classification. J Gastrointest Surg 15:1969–1976
Disclosures
Drs. Nakata, Nagai, Ohuchida, Shimizu, and Tanaka have no conflicts of interest of financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakata, K., Nagai, E., Ohuchida, K. et al. Technical feasibility of laparoscopic total gastrectomy with splenectomy for gastric cancer: clinical short-term and long-term outcomes. Surg Endosc 29, 1817–1822 (2015). https://doi.org/10.1007/s00464-014-3870-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3870-6