Abstract
Background
The uptake of minimally invasive oesophagectomy (MIO) in the UK has increased dramatically in recent years. Post-oesophagectomy diaphragmatic hernias (PODHs) are rare, but may be influenced by the type of approach to resection. The aim of this study was to compare the incidence of symptomatic PODH following open and MIO in a UK specialist centre.
Methods
Consecutive patients undergoing oesophagectomy for malignant disease between 1996 and 2012 were included. A standardised, radical approach to the abdominal phase was employed, irrespective of the type of procedure undertaken. Patient demographics, details of surgery and post-operative complications were collected from patient records and a prospective database.
Results
A total of 273 oesophagectomies were performed (205 open; 68 MIO). There were 62 hybrid MIOs (laparoscopic abdomen and thoracotomy) and six total MIOs. Seven patients required conversion and were analysed as part of the open cohort. Nine patients (13.2 %) developed a PODH in the MIO cohort compared with two patients (1.0 %) in the open cohort, (p < 0.001). Five patients developed hernias in the early post-operative period (days 2–10): all following MIO. Both PODHs in the open cohort occurred following transhiatal oesophagectomy. All PODHs were symptomatic and required surgical repair. CT thorax confirmed the diagnosis in 10 patients. Seven hernias were repaired laparoscopically, including two cases in the early post-operative period. PODHs were repaired using the following techniques: suture (n = 6), mesh reinforcement (n = 4) and omentopexy to the anterior abdominal wall without hiatal closure (n = 1). There were two recurrences (18 %).
Conclusions
The incidence of symptomatic PODH may be higher following MIO compared to open surgery. The reasons for this are unclear and may not be completely explained by the reduction in adhesion formation. Strategies such as fixation of the conduit to the diaphragm and omentopexy to the abdominal wall may reduce the incidence of herniation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Oesophagectomy is a high risk procedure that subjects the patient to a significant physiological insult. Data from the National Oesophago-gastric Cancer Audit 2013 showed that post-operative inpatient morbidity following oesophagectomy in the United Kingdom (UK) was 32 % with a 30-day mortality of 1.7 % [1]. Evidence suggests that minimally invasive oesophagectomy (MIO) techniques are associated with a reduction in morbidity, length of stay, blood loss and quality of life compared to open oesophagectomy [2, 3]. These potential benefits, coupled with the technological advancements in minimally invasive technologies, have lead to the increased uptake of MIO in recent years. Currently, MIOs represent 43 % of all oesophagectomies performed in the UK, of which two-thirds are performed using a hybrid technique via a laparoscopic approach to the abdomen and an open thoracotomy [1].
Diaphragmatic herniation of the abdominal contents through the hiatus and into the thoracic cavity is a rare but potentially fatal complication of oesophagectomy. The reported incidence of post-oesophagectomy diaphragmatic herniation (PODH) in contemporary series of MIOs ranges from 2.2 to 26 % [4–10]. The incidence of PODH following open oesophagectomy appears to be lower with reported rates of 0.2–6.0 % [5, 6, 8, 9, 11–18]. The reasons for this difference remain unclear, but may be related in part to a reduction in intra-abdominal adhesions in MIOs. Furthermore, there is little consensus on how cases of PODH should be managed in terms of indications for surgery, choice of approach and methods of repair. Data from the UK regarding both the incidence and management of PODH are lacking with most reports in the literature limited to high volume centres in North America. Therefore, the objectives of this study were (1) to compare the incidence of symptomatic PODH between open oesophagectomy and MIOs at our own centre and (2) to analyse the treatment strategies employed in patients who developed PODH.
Materials and methods
From January 1996 to December 2012, data from 273 consecutive patients undergoing oesophagectomy at Gloucestershire Royal Hospitals NHS Foundation Trust were entered into an institution approved, prospectively maintained database. Only patients undergoing oesophagectomy for malignancy or high grade dysplasia were included. Data were collected on age, gender, histopathologic diagnosis, use of neoadjuvant therapy, American Society of Anaesthesiologists (ASA) grade, surgical approach, complications and survival.
Until October 2005, open oesophagectomies were exclusively performed using the following techniques: two-stage Ivor-Lewis (through a right thoracotomy), transhiatal (with cervical anastomosis) and three-stage McKeown (abdominal, thoracic and cervical approaches). The choice of technique was based on the anatomic location of the lesion and surgeon preference. MIO was introduced in October 2005. A standardised procedure was adopted involving a laparoscopic approach to the abdomen for formation of the gastric conduit and feeding jejunostomy and a right-sided thoracotomy to complete the resection and perform the anastomosis. A radical approach to the abdominal phase was employed irrespective of the technique used to perform the oesophagectomy. This involved division of the left gastric artery at its origin, lymphadenectomy along the hepatic and splenic arteries, a wide crural resection taking a sufficient cuff of crural fibres and diaphragm to minimise the risk of a positive circumferential margin, resection of the pericardial fat pad and opening of the pleural cavities (occasionally deferred until the thoracic phase). No attempt was made to fix the gastric conduit to the diaphragm or crura for either an open or MIO. Suture apposition of the crura was reserved for cases of an excessively widened hiatus. MIO was the preferred method of oesophagectomy for four of the six surgeons who contributed cases over the time period of this study. There were no absolute contraindications to MIO, although previous open upper abdominal surgery was considered a relative contraindication. Of the four surgeons performing MIO, one surgeon had received formal training in MIO as a surgical trainee, with the other three surgeons having extensive experience in minimally invasive surgery prior to the adopting MIO. While no formal external preceptorship was instituted, the unit practised dual surgeon operating for MIO cases whenever possible. In 2006, Gloucestershire Royal Hospitals NHS Foundation Trust became a designated upper gastrointestinal resection centre resulting in an increase in the mean number of oesophagectomies from 10.1 to 24.6 per year.
PODH was defined as herniation of the intra-abdominal contents, other than the gastric conduit, through the hiatus. For the purposes of this study, an early PODH was defined as arising ≤30 days post-resection and a late PODH as arising >30 days post-resection. Typically, surgical follow-up of patients was every 3 months in the first post-operative year, at 6 month intervals for the next 2 years and annually thereafter. Computerised tomography (CT) was not routinely undertaken as a means of surveillance and was only performed in those patients who were symptomatic or were receiving adjuvant therapy. Therefore, the incidence of symptomatic PODH was reported by this study.
Data are presented as frequencies, means with standard deviations or percentages. Differences in baseline clinicopathologic features and post-operative outcomes were compared using Student’s t test, chi-squared test, Fisher’s exact probability test and Mann–Whitney U test where appropriate. Mortality rates were adjusted for baseline differences between the open and MIO cohorts by means of regression analysis. A p value of ≤0.05 was considered statistically significant.
Results
Of the 273 patients who underwent oesophagectomy, an MIO was attempted in 75 cases (27.0 %). Seven patients required conversion to an open Ivor-Lewis procedure (9.3 %) and were analysed as part of the open cohort. Six patients underwent an MIO via a laparoscopic abdominal and thoracoscopic approach with cervical anastomosis. The proportion of patients undergoing an MIO increased from 8.3 % in 2006 to 82.6 % in 2012. Among patients who underwent open oesophagectomy (n = 205), an Ivor-Lewis was performed in 144 cases (70.3 %), transhiatal in 42 cases (20.5 %) and McKeown in 19 cases (9.3 %).
Patients undergoing MIO were older (64.0 vs. 61.3 years, p = 0.042), were more likely to have received neoadjuvant chemotherapy (83.8 vs. 54.1 %, p < 0.001), were more likely to have tumours that were adenocarcinomas (92.6 % vs. 80.5 %, p = 0.013) and had tumours of a less advanced stage (p = 0.009) (Table 1). There was no difference in the median post-operative length of stay between the MIO and open cohorts [14.5 days (range 8–93 days) vs. 15.0 days (range 9–195 days) (p = 0.472)]. Following correction for baseline differences between the MIO and open cohorts, there were no significant differences in either the 30- or 90-day mortality rates. In the period covered by National Oesophago-gastric Cancer Audit 2013, both the 30- and 90-day mortality rates were zero.
A PODH was identified in nine patients in the MIO cohort (13.2 %) compared with two patients in the open cohort (1.0 %) (p < 0.001). One patient who developed a PODH in the MIO cohort had undergone a laparoscopic and thoracoscopic resection with a cervical anastomosis. Both patients who developed a PODH in the open cohort had undergone a transhiatal oesophagectomy. All eleven patients with a PODH were symptomatic and required surgical repair. The details of these patients are summarised in Table 2. Five patients developed hernias in the early post-operative period (days 2–10), each of whom had undergone an MIO. The median time from resection to repair of a late PODH was 16.5 months (range 6–114 months). A CT scan was used to confirm the diagnosis in 10 cases with a chest radiograph used as the sole diagnostic modality in one case of an early PODH. Acute respiratory compromise and chest pain were the main presenting features of the patients who developed an early PODH, whereas worsening dyspnoea and vague epigastric or chest discomfort were reported by patients who developed late PODHs. In one case of an early PODH, the patient was readmitted on day 10 with a right-sided chylothorax that was managed non-operatively with a tube thoracostomy and fat-restricted nasojejunal feeding supplemented with medium-chain fatty acids. A CT thorax on readmission revealed a co-existing PODH with herniation of the transverse colon into the left hemithorax. Interestingly, a previous CT thorax that had been performed on day 7 to exclude an anastomotic leak, revealed a small PODH that had not been recognised at the time of the original radiological report.
At repair, transverse colon was identified in the hernial sac in ten patients, four of whom had co-existing herniation of the small bowel. Only one patient had a hernia that contained small bowel alone. None of the patients who developed a PODH required bowel resection for ischaemia. In every case of PODH, the hernia passed to the left of the gastric conduit and into the left hemithorax. All hernias were successfully reduced via an abdominal approach. In eight patients who underwent an MIO, a laparoscopic repair was attempted with one patient requiring conversion to an open procedure to facilitate manual reduction of the hernia. One patient required an open repair of a PODH following MIO due to cardiopulmonary instability and to permit suture ligation of the thoracic duct for chylothorax. In four of the five patients with an early PODH, suture repair alone was performed due to concerns about the potential for a mesh repair to compromise the vascularity of the gastric conduit. All late PODHs that occurred following MIO were repaired laparoscopically using a porcine mesh (SURGISIS, Cook Surgical, Bloomington, Indiana, USA) to reinforce the suture closure of the hiatal defect (Fig. 1A–C). Both cases of PODH following open oesophagectomy were repaired via an open approach. In one case, a giant hiatal defect was deemed too large to close, and instead, an omentopexy was performed with fixation of the omentum to the anterior abdominal wall. There were no post-operative deaths following repair. The median follow-up was 22 months (range 7–122 months). All patients who underwent repair of a PODH remain alive. There were two recurrences, occurring at an interval of nine and 12 months after the initial repair, respectively. One recurrence developed following laparoscopic mesh repair of a late PODH and was repaired via a laparoscopic approach with another porcine mesh. The second patient developed a recurrence following an open suture repair of a late PODH and was repaired with a mesh via an open approach. To date, neither patient has developed a re-recurrent hernia.
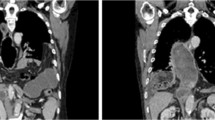
Diaphragmatic hernia of patient 4 from Table 2. A Transverse CT image showing herniation of the transverse colon into the left chest (see arrow), B Transverse colon can be seen herniating antero-laterally to the gastric conduit, C Porcine mesh (SURGISIS; Cook Surgical, Bloomington, Indiana, USA) suture fixation with spiral tacks to the crura following anterior closure of the hiatal defect
Discussion
The increased uptake in MIO appears to be mirrored by an increase in reports of PODH in the literature. To date, there have been 231 reports of PODH, the majority of which have been reported in the last 5 years. Determining the true incidence of PODH is difficult, owing to the considerable heterogeneity in operative approach, post-operative surveillance protocols and duration of follow-up. Nevertheless, there is a growing body of evidence to suggest that the rate of PODH is higher in patients following MIO, with a combined mean of 5.2 %, compared to a combined mean rate of 0.8 % in patients following open oesophagectomy (Tables 3, 4).
This study is unique in that it is the first UK centre to demonstrate that the rate of PODH appears to be higher following MIO. It is also the second largest worldwide series reporting experience with PODH repair. Data published in abstract form from another specialist centre in the UK suggests that our experience with PODH following MIO is not an isolated phenomenon [20]. However, PODH is not a specific data item captured by the National Oesophago-gastric Cancer Audit, thus making it impossible to determine the rate of PODH at upper gastrointestinal resection centres across the UK.
The PODH rate of 13.2 % following MIO in this study appears high, although several recent studies report a PODH rate of similar magnitude following their initial experience with MIO. The PODH rate following open oesophagectomy (1.0 %) is comparable to rates reported elsewhere in the literature. This study differs in two key aspects regarding the nature of the PODHs that developed. First, all of the PODHs were symptomatic and required repair, and second, more than half of the PODHs following MIO occurred in the early post-operative period.
In this study, CT was not undertaken as a routine means of surveillance and was only reserved for those patients who were symptomatic. In a dedicated review of post-operative surveillance CT scans, Ganeshan et al. [9] demonstrated a PODH rate of 15 % in a series of predominantly open oesophagectomies. Based on these findings, the true incidence of PODH in the current study may have been much higher. Interestingly, only 13 % of patients with a PODH in the study by Ganeshan et al. underwent repair, as the majority were asymptomatic. Furthermore, only 10 % of their cases were detected by the radiologist on initial prospective review, with one-third of hernias containing intra-abdominal fat alone [9]. In contrast, only one PODH in the current study had been missed on a previous CT scan and all hernias were symptomatic, containing either transverse colon or small bowel. Based on our findings and the available evidence, it is difficult to define the exact indications for repair of a PODH. A pragmatic approach should be adopted where due consideration is given to patient symptoms, fitness, the presence of recurrent disease, the size and contents of the hernia sac.
Review of the literature suggests that almost 90 % of PODHs following MIO occur in the late post-operative period (Table 4). The most likely explanation relates to a reduction in intra-abdominal adhesion formation that may limit the mobility of the abdominal viscera, although this may not entirely account for the increased incidence of early relative to late PODHs in this series. Potential causes of early PODH remain speculative, but warrant discussion. All oesophagectomies were performed for suspected invasive disease and as such merited a wide dissection of the hiatus. It is possible that the view afforded by laparoscopy was in some way distorted by the pressure of the pneumoperitoneum, prompting the surgeon to perform a more radical dissection that weakened the tissues and predisposed to herniation. The distance to the circumferential radial margin, and to a lesser extent lymph node yield, may act as surrogate markers for radicality of resection, although these were not routinely recorded in the database. Formation of the feeding jejunostomy was performed laparoscopically and necessitated retraction of the transverse colon superiorly in order to facilitate identification of the duodenojejunal flexure. The omentum may have become lodged at the hiatus from where it could have acted as a lead point for herniation of the transverse colon into the chest.
At the time of repair, all PODHs were found to have occurred to the left of the conduit, consistent with the findings of previous studies where right-sided herniation has rarely been noted [5, 6]. One theory suggests that the staple line along the lesser curve of the gastric conduit promotes adhesion formation to the right crus more readily than that of the smooth, serosal surface of the greater curve to the left crus, thus preventing herniation of the intra-abdominal viscera. In addition, the left lobe of the liver may also act as a mechanical barrier. The methods used to repair PODHs in this study were dictated by approach to the abdomen at oesophagectomy, the urgency of presentation and surgeon preference. Our experience indicates that it is safe to perform a laparoscopic repair of a PODH following MIO, even in the acute setting as long as the patient does not experience significant cardiopulmonary compromise. It is important to remember that not all hernias can be repaired via an abdominal approach, either laparoscopic or open, and that the surgeon should be prepared to undertake a thoracotomy in cases where intrathoracic adhesions prevent abdominal reduction of the contents. The key to any successful hernia repair is a tension-free closure, which is best achieved by complete mobilisation of the crura prior to the suture apposition. PODH repair is a particularly high risk procedure as achieving a snug closure around the conduit has to be weighed against the potential for vascular compromise. Evidence for the superiority of mesh reinforcement over suture apposition alone for primary hiatus hernia is limited to two small-scale randomised controlled trials that demonstrate a reduction in short-term recurrence rates [21, 22]. Many surgeons still have concerns over the potential for visceral erosion and it is for this reason that mesh placement was avoided in the majority of early PODHs where the need for an adequately vascularised conduit was at its most crucial. If tension-free closure cannot be achieved due to the size of the hiatal defect, then omentopexy alone may suffice. The recurrence rate of 18 % in this study compares favourably to other series where rates of 13–44 % have been reported with a similar length of follow-up [6, 9, 19].
Given the increased incidence of PODH with MIO, several centres have recommended suture fixation of the conduit to the crura. There has been no direct comparison of PODH rates between conduit fixation and non-fixation, although centres who perform routine fixation report rates of 2.8–7.9 % [6, 10]. Placement of the sutures can be technically challenging and care must be taken to avoid disruption of the right gastro-epiploic artery. We have found that conduit fixation is best achieved at thoracotomy, but have now moved towards a less demanding modified omentopexy where the omentum is split and fixed to the anterior abdominal wall at the time of jejunostomy formation. There have been no PODHs following MIOs in 2013, although it would be premature to hail this technique as a success without long-term follow-up.
The main limitation of this study was the limited number of outcome events, which prevented a more accurate comparison of the open and MIO cohorts by adjustment for differences in baseline clinicopathological features. There was also considerable heterogeneity due to different surgeons and surgical techniques. Despite these limitations, it appeared that a minimally invasive approach was the key risk factor for development of a PODH. It could be argued that the number of MIOs was relatively small compared to other published series. However, this study is important in that it incorporates our initial experience with a procedure where the learning curve is not well defined and may in fact be more reflective of similar volume centres across the UK.
In conclusion, the incidence of symptomatic PODH may be higher following MIO compared to the open approach. The reasons for this remain unclear and may not be completely explained by the reduction in adhesion formation. Laparoscopic repair, even in the acute setting, is feasible, although the benefits of mesh reinforcement need to be weighed against the risk of vascular compromise to the conduit. Pooled analysis of outcome data from multiple centres is required to obtain a more accurate picture of PODH rates. Future studies should focus on the impact of strategies, such as conduit fixation and omentopexy, on PODH rates and whether there is any benefit from serial CT surveillance.
References
The Royal College of Surgeons of England (2013) National Oesohago-Gastric Cancer Audit 2013. An audit of the care received by people with oesophago-gastric cancer in England and Wales. http://www.rcseng.ac.uk/media/docs/press_releases/national-oesophago-gastric-cancer-audit-2013. Accessed 27 March 2014
Nagpal K, Ahmed K, Vats A, Yakoub D, James D, Ashrafian H et al (2010) Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 24:1621–1629
Biere S, van Berge Henegouwen M, Maas K, Bonavina L, Rosman C, Garcia J et al (2012) Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 379:1887–1892
Fumagalli U, Rosati R, Caputo M, Bona S, Zago M, Lutmann F, Peracchia A (2006) Diaphragmatic acute massive herniation after laparoscopic gastroplasty for esophagectomy. Dis Esophagus 19:40–43
Vallböhmer D, Hölscher AH, Herbold T, Gutschow C, Schröder W (2007) Diaphragmatic hernia after conventional or laparoscopic-assisted transthoracic esophagectomy. Ann Thorac Surg 84:1847–1853
Kent MS, Luketich JD, Tsai W, Churilla P, Federle M, Landreneau R, Alvelo-Rivera M, Schucher M (2008) Revisional surgery after esophagectomy: an analysis of 43 patients. Ann Thorac Surg 86:975–983
Sutherland J, Banerji N, Morphew J, Johnson E, Dunn D (2011) Postoperative incidence of incarcerated hiatal hernia and its prevention after robotic transhiatal esophagectomy. Surg Endosc 25:1526–1530
Willer BL, Worrell SG, Fitzgibbons RJ Jr, Mittal SK (2012) Incidence of diaphragmatic hernias following minimally invasive versus open transthoracic Ivor Lewis McKeown esophagectomy. Hernia 16:185–190
Ganeshan DM, Correa AM, Bhosale P, Vaporciyan AA, Rice D, Mehran RJ, Walsh GL, Iyer R, Roth JA, Swisher SG, Hofstetter WL (2013) Diaphragmatic hernia after esophagectomy in 440 patients with long-term follow-up. Ann Thorac Surg 96:1138–1145
Bronson NW, Luna RA, Hunter JG, Dolan JP (2014) The incidence of hiatal hernia after minimally invasive esophagectomy. J Gastrointest Surg. doi:10.1007/s11605-014-2481-9
Barbier PA, Luder PJ, Schupfer G, Becker CD, Wagner HE (1988) Quality of life and patterns of recurrence following transhiatal esophagectomy for cancer: results of a prospective follow-up of 50 patients. World J Surg 12:270–276
Katariya K, Harvey JC, Pina E, Beattie EJ (1994) Complications of transhiatal esophagectomy. J Surg Oncol 57:157–163
Heitmiller RF, Gillinov AM, Jones B (1997) Transhiatal herniation of colon after esophagectomy and gastric pull-up. Ann Thorac Surg 63:554–556
Orringer MB (1996) Transhiatal esophagectomy without thoracotomy. In: Orringer MB, Zuidema GD (eds) Shackelford’s surgery of the alimentary tract, vol 1, 4th edn. WB Saunders, Philadelphia, pp 414–445
Van Sandick JW, Knegjens JL, van Lanschot JJB, Obertop H (1999) Diaphragmatic herniation following oesophagectomy. Br J Surg 86:109–112
Franceschi A, Mariette C, Balon JM, Fabre S, Triboulet JP (2002) Hernie diaphragmatique après oesophagectomie: à propos de deux cas et revue de la literature. Ann Chir 127:62–64
Liu JF, Wang QZ, Ping YM, Zhang YD (2008) Complications after esophagectomy for cancer: 53-year experience with 20,796 patients. World J Surg 32:395–400
Daiko H, Nishimura M, Hayashi R (2010) Diaphragmatic herniation after esophagectomy for carcinoma of the oesophagus: a report of two cases. Esophagus 7:169–172
Price TN, Allen MS, Nichols FC, Cassivi SD, Wigle DA, Shen KR, Deschamps C (2011) Hiatal hernia after esophagectomy: analysis of 2,182 esophagectomies from a single institution. Ann Thorac Surg 92:2041–2045
Nicholas R, Guarino S, Qureshi Y, Hughes F (2013) Diaphragmatic hernia following laparoscopically-assisted Ivor-Lewis oesophagectomy: experience from 206 consecutive oesophagectomies. Br J Surg 100(Suppl 8):5A
Frantzides CT, Madan AK, Carlson MA, Stavropoulus GP (2002) A prospective randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs. simple cruroplasty for large hiatal hernia. Arch Surg 137:649–652
Granderath FA, Schweiger UM, Kamolz T, Asche KU, Pointer R (2005) Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg 140:40–48
Disclosures
David Messenger, Simon Higgs, Simon Dwerryhouse, David Hewin, Mark Vipond, Hugh Barr and Martin Wadley have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Messenger, D.E., Higgs, S.M., Dwerryhouse, S.J. et al. Symptomatic diaphragmatic herniation following open and minimally invasive oesophagectomy: experience from a UK specialist unit. Surg Endosc 29, 417–424 (2015). https://doi.org/10.1007/s00464-014-3689-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3689-1