Abstract
Objective
To evaluate the safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors (GISTs) of the stomach with systematic review and meta-analysis.
Methods
The literature database before March, 2014 was extensively searched to retrieve the comparative studies of laparoscopic (LAP) and open resection (OPEN) for GISTs with a relevance of study goal. The inclusion and exclusion criteria were formulated. After a quality evaluation, the data were extracted. The Cochrane collaboration RevMan5.1 version software was used for meta-analysis.
Results
There are nineteen studies meeting the inclusion criteria for meta-analysis. The total sample size of these studies was 1,060 cases. The operation time was similar between the two groups [weighted mean difference (WMD) −7.20 min, 95 % confidence interval (CI) −25.65 to 11.25, P = 0.44)]. Compared to OPEN, however, LAP experienced less blood loss (WMD −54.21 ml, 95 % CI −82.65 to −25.77, P < 0.01), earlier time to flatus (WMD −1.34 days, 95 % CI −1.62 to −1.06, P < 0.01) and oral diet (WMD −1.80 days, 95 % CI −2.18 to −1.42, P < 0.01), shorter hospital stay (WMD −3.68 days, 95 % CI −4.52 to −2.85, P < 0.01) and decrease in overall complications [relative risk (RR) 0.51, 95 % CI 0.32–0.80, P < 0.01)]. In addition, the long-term follow-up result shows that there is no significant difference in the two groups of patients.
Conclusion
Laparoscopic resection for gastric GISTs is a safe and feasible procedure with less blood loss, less overall complications and quicker recovery. The long-term survival situation of patients mainly depends on the tumor itself risk, and laparoscopic surgery will not increase the risks of tumor relapse and metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastrointestinal stromal tumors (GISTs), which are often characterized by high expression of KIT [1, 2], are the most common mesenchymal tumor in the gastrointestinal tract. GISTs most frequently occurs in the stomach (60 %) in the form of submucosal tumors, followed by jejunum or ileum (30 %), duodenum (5 %), colon and rectum (<5 %), esophagus (<1 %) and appendix (<1 %) [2]. GISTs have malignant potential, and it is reported that recurrence of GISTs often occurs at the peritoneal surface or liver [3]. Because gastric gastrointestinal stromal tumor is not completely distinguished from other submucosal tumors, a surgical excisional biopsy is recommended for tumors >2 cm. The surgical principles of gastrointestinal stromal tumor are composed of an R0 resection with a normal mucosa margin, no systemic lymph node dissection and avoidance of rupture, which results in peritoneal seeding even in cases with otherwise low risk profiles.
Since the development of minimally invasive surgical approaches, laparoscopic surgery (LAP) for gastrointestinal tumors has evolved rapidly over the past decade. Various types of laparoscopic approaches for GISTs have been described, including wedge resection of the stomach, intragastric tumor resection and combined endoscopic-laparoscopic resection [4–7]. Several case series have proved the safety and feasibility of LAP for gastric GISTs; however, the oncologic benefits of LAP for GISTs have not been widely reported and the sample size of those researches were relatively small. Furthermore, there is no randomized controlled trials (RCTs) yet, which compares outcomes between LAP and the open approach (OPEN). Lack of RCTs may be because of the difficulty encountered in conducting a large RCT in clinical practice. Therefore, we present a systematic review of the literature and a comparative effectiveness analysis of LAP versus OPEN. Using meta-analytic techniques, we set out to evaluate the surgical and oncologic outcomes of patients undergoing either procedure as reported in the published literature.
Methods
Search strategy
Systematic searches of PubMed, Embase, Cochrane Library, and Web of Science were performed to identify articles published up to March 2014 that compared LAP and OPEN. The search terms “gastrointestinal stromal tumor”, “GIST”, “laparoscopic”, “laparoscopy”, “gastrectomy” and “gastric resection” were utilized. The links of every search result and all references in the original articles identified were reviewed to identify the additional literature that was not indexed. The language of the articles was limited to English and Chinese according to the reviewers’ language competence.
Eligibility criteria
Studies meeting the following criteria were included: Comparative, peer-reviewed studies of LAP versus OPEN for patients with GISTs for which the full text of the article was available. If two studies from the same group were identified, the most recent study or that including more subjects was selected unless the reports were from different time periods. The papers containing any of the following were excluded: (1) tumors out of the stomach such as jejunum or ileum; (2) studies in which <2 interested indexes were reported, or it was difficult to calculate these from the results.
Data extraction and quality assessment
Two authors independently extracted the data using a unified datasheet, and decided upon the controversial issues through discussion. Extracted data included the following: author, study period, geographical region, number of patients, operation time, blood loss, time to flatus, time to oral intake, length of hospital stay, morbidity, mortality and long-term outcomes. Postoperative complications were classified as medical (cardiovascular, respiratory, or metabolic events; nonsurgical infections; deep venous thrombosis; and pulmonary embolism) or surgical (any anastomotic leakage or fistula, any complication that required reoperation, intra-abdominal collections, wound complications, bleeding events, pancreatitis, ileus, delayed gastric emptying, and anastomotic stricture). This classification system is based on the Memorial Sloan–Kettering Cancer Center complication reporting system [8]. If the study provided medians and ranges instead of means and standard deviations (SDs), we estimated the means and SDs as described by Hozo et al. [9]. The qualities of the included studies were assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS). This scale varies from zero to nine stars: Studies with a score equal to or higher than six were considered methodologically sound.
Statistical analysis
Continuous variables were assessed using weighted mean difference (WMD), and dichotomous variables were analyzed using the risk ratio (RR). Statistical heterogeneity, which indicated between-study variance, was evaluated according to the Higgins I 2 statistic [10]. To account for clinical heterogeneity, which refers to diversity in a sense that is relevant for clinical situations, we used the random-effects model based on DerSimonian and Laird’s method. We hypothesized the outcomes of the comparison may be affected by the uneven distribution of the surgical types between the LAP and OPEN groups, especially by the relatively larger proportion of extended surgeries performed in the OPEN group. Thus, we performed a subgroup analysis of patients who underwent wedge resection in the two groups to eliminate the bias from the surgical type selection. We also conducted a subgroup analysis of studies which had comparable tumor size or risk index because the learning curve may have an impact on the operative outcomes. Potential publication bias was determined by conducting informal visual inspection of funnel plots based on the complications. Data analyses were performed using Review Manage version 5.1 (RevMan 5.1) software downloaded from Cochrane Library. P < 0.05 was considered statistically significant.
Results
Studies selected
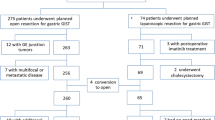
A total of 628 citations were obtained from searches of the various electronic bibliographies. After the titles and abstracts were reviewed, papers without comparison of LAP and OPEN were excluded, which left 24 comparative studies, five [11–15] of which did not meet the inclusion criteria and were excluded. This left a total of nineteen observational studies [16–34], all of which were accessible in full-text format. Eighteen studies were published in English and one in Chinese. A flow chart of the search strategies, which contains reasons of excluded studies, is illustrated in Fig. 1.
Study characteristics and quality
A total of 1,060 patients were included in the analysis with 516 undergoing LAP (48.7 %) and 544 undergoing OPEN (51.3 %). They represented an international experience including data from 10 different countries or regions (4 Japan, 4 United States, 3 China, 2 Korea, 1 United Kingdom, 1 Italy, 1 Belgium, 1 Austria, 1 Singapore and 1 Taiwan). Table 1 presents the characteristics of the included studies, whereas Table 2 presents the quality assessment based on the NOS. In general, the quality of the included studies was satisfactory. According to the NOS, three out of the nineteen observational studies got 6 stars, eight articles got 7 stars, three articles got 8 stars and the remaining five got 9 stars.
Evidence from primary outcomes
Eighteen studies reported operation time [16–22, 24–34]. The present analysis showed no statistically significant difference in the operation time of the two groups (WMD −7.20 min; 95 % CI −25.65 to 11.25; P = 0.44) (Fig. 2). Twelve studies reported blood loss [16, 17, 19, 20, 24–26, 28, 30, 31, 33, 34]. Intraoperative blood loss was significantly lower in the LAP compared with the OPEN group (WMD −54.21 ml; 95 % CI −82.65 to −25.77 ml; P < 0.01) (Fig. 3). The outcomes also favored LAP in first flatus day (WMD −1.34 days; 95 % CI −1.62 to −1.06, P < 0.01) (Fig. 4) and first oral intake (WMD −1.80 days; 95 % CI −2.18 to −1.42, P < 0.01) (Fig. 5), which indicated a quicker recovery of the bowl function. Three studies reported shorter duration or the lower dosage of analgesic application after LAP [18, 24, 26]. Moreover, postoperative hospital day was 3.68 days shorter for LAP patients (WMD −3.68 days; 95 % CI −4.52 to −2.85, P < 0.01) (Fig. 6).
The rate of overall postoperative complications was significantly lower for LAP (RR 0.51, 95 % CI 0.32–0.80, P < 0.01) (Fig. 7). Visual inspection of the funnel plot revealed symmetry, indicating no serious publication bias (Fig. 8). After further analysis, surgical complications were similar between the two groups (RR 0.71, 95 % CI 0.36–1.39, P = 0.31). However, LAP was associated with a marginal reduction in medical complications (RR 0.53, 95 % CI 0.28–1.02, P = 0.06). The specific postoperative complications included in the studies are summarized in Table 3.
Seventeen studies reported tumor size [16–20, 22–27, 29–34]. The tumor size for LAP was significantly smaller than that for OPEN from the analysis of 1,001 resections (WMD −0.93 cm; 95 % CI −1.33 to −0.53, P < 0.01) (Fig. 9).
During the follow-up period, tumor recurrence was observed in twelve studies [17, 18, 20, 22–28, 30, 32]. The recurrence risk in LAP was 3.3 % (11/330) and 9.3 % (32/345) in OPEN, and patients who underwent LAP were less likely than the OPEN to have recurrence (RR 0.47, 95 % CI 0.24–0.93, P = 0.03) (Fig. 10). The available data about recurrence patterns, specific recurrent sites and survival outcomes are summarized in Table 4.
Subgroup analysis for studies of wedge resection
Seven studies used only wedge resection in both LAP and OPEN group [16, 18, 19, 22, 24, 26, 29]. Another one study provided a subgroup analysis of wedge resection with adequate data [30]. We also used it for pooled analysis. The overall effects such as operation time, blood loss, time to flatus or oral intake, hospital stay, complications and tumor size remained unchanged in subgroups. However, in this subgroup analysis, the recurrence risk in LAP was 5.4 % (7/130) and 5.5 % (9/165) in OPEN, and the difference was not significant (RR 1.01, 95 % CI 0.39–2.63, P = 0.99). The outcomes of subgroup analysis for studies of wedge resection are summarized in Table 5.
Subgroup analysis for studies with comparable tumor size or risk index
Eleven studies were qualified for this subgroup analysis [16, 17, 19, 20, 22, 25, 26, 29, 30, 33, 34]. Like the subgroup analysis for wedge resection, outcomes other than tumor recurrence remained unchanged. And the recurrence risk was similar between LAP and OPEN (RR 0.69, 95 % CI 0.30–1.60, P = 0.39). The outcomes of subgroup analysis for studies with comparable tumor size or risk index are summarized in Table 6.
Discussion
GISTs are uncommon mesenchymal tumors that arise in the wall of the gastrointestinal tract. The advent of imatinib mesylate significantly reduces the recurrence rate of GISTs, but surgery remains the mainstay of therapy for primary GISTs with no evidence of metastasis. Laparoscopic surgery is increasingly performed for surgical treatment of gastric GISTs. Although RCTs are the most ideal tool for meta-analysis, there have been no RCTs comparing laparoscopic surgery with open surgery for gastric GISTs. This may be due to the difficulties to conduct a high-quality RCT to evaluate a new surgical intervention because of obstacles such as learning curve effects, ethical and cultural resistance, urgent or unexpected conditions during the operation and the relatively low incidence. Therefore, due to the unavailability of RCTs, inclusion of non-RCTs is an appropriate strategy to extend the source of evidence. In order to assess the efficacy and safety of laparoscopic surgery for gastric GISTs, we extracted relative data as much as possible and we pooled the outcome whenever possible.
The operative time in the LAP group was not longer than OPEN which is different from many other types of gastrointestinal surgery [35–37]. Because of the low frequency of lymph node metastasis, local resection of the tumor with a disease-free margin is recommended and lymphadenectomy, which is time-consuming under laparoscopy, is not generally required. As time spent on the establishment of pneumoperitoneum and the closure of the trocar incision and mini-laparotomy is likely to be shorter than the opening and closure of laparotomy, it might explain the possible fact that the LAP to be shorter than OPEN with the development of the surgical techniques and laparoscopic instruments. Operative blood loss was shown in the pooled analysis to be lower in LAP cases. The reduced length of incision wound and the application of energy-dividing devices, such as the Harmonic Scalpel and LigaSure, contribute to the reduction in blood loss. Another reason is that laparoscopy allows for the magnified view of small vessels. However, this result should be interpreted prudently for the variation in blood loss between studies was high, with heterogeneity as a result of different methods of estimating blood loss. Besides, some included studies selected patients with smaller tumors in LAP group [16, 18, 21, 23, 27, 28, 31, 32, 34] or more extensive gastrectomy or higher additional resection rate in OG group [17, 23, 27–32]. Lack of adequate matching in such results makes comparison of operative blood loss inherently flawed and at a high risk for confounding.
The postoperative morbidity is usually used to estimate the feasibility and safety of a procedure. The meta-analysis demonstrated a reduced number of complications in the LAP versus OPEN group, which may have resulted from a reduction in medical complications. It was conceivable that surgical complications were similar between groups because LAP results in the same resection extent as OPEN. And the marginally decreased medical complications could be explained by the reduced invasiveness of the laparoscopic technique and less postoperative pain. Pain after surgery was less serious in LAP than in OPEN surgery due to the shorter duration or the lower dosage of analgesic application [18, 24, 26]. The pain caused by large incision as well as the use of tension sutures and abdominal bandages after laparotomy can make it difficult for patients to cough, expectorate and perform breathing exercise effectively, thus leading to complications such as pulmonary infection [38]. Our pooled analysis demonstrated the postoperative hospital day was 3.68 days shorter for LAP patients. Reduced use of analgesic drugs, shortened time of abdominal cavity exposure, less bowel manipulation, alleviated inflammatory reactions and earlier postoperative activities are considered to be the main reasons for earlier gastrointestinal recovery from laparoscopic surgery.
Long-term survival remains critical for all patients with GISTs regardless of a benign or malignant designation since these tumors have an uncertain biologic behavior. Our pooled analysis of primary data demonstrated that postoperative recurrence in LAP group was less than that of OPEN group, and the difference was statistically significant. However, in part of the included literatures, the diameter of tumor in OPEN group was larger than that in LAP group, or the risk classification was higher than LAP group. It is widely accepted that the tumor size and mitotic index are two key factors on GISTs long-term outcomes. Thus, the literatures with the same surgical approach (wedge resection) [16, 18, 19, 22, 24, 26, 29] as well as the literatures with comparable tumor size or risk classification were given subgroup analysis [16, 17, 19, 20, 22, 25, 26, 29, 30, 33, 34]. However, the results of two subgroup analysis still showed that the risk of postoperative recurrence in LAP group was not higher than OPEN group. In addition, it can be seen from Table 4 that the common sites of postoperative recurrence of GISTs included liver metastasis, peritoneal metastasis and local recurrence. It was not hard to find that the vast majority of cases of recurrence or metastasis were patients with high-risk classification, which was not clearly related to the operative grouping [17, 18, 20, 22, 23, 25, 27, 32]. Therefore, it was believed that with the continuous advances in the technology, as long as the surgeon strictly select the proper case, strictly follow the radical principles of tumor surgery for complete resection and avoid tumor ruptures, LAP could achieve a long-term effect almost the same with laparotomy in addition to its advantage of minimally invasive.
There are several limitations to our studies. First, all of the studies included in this meta-analysis are non-RCTs, such as clinical controlled trial, prospective or retrospective cohort and as a result of study design limitations, and these studies were more likely to suffer from various kinds of bias. Furthermore, confounding factors which were balanced by randomization in RCTs often disturbed the observation of effect of the intervention in NRTs. The allocation concealment was not described in the included studies, which played an equally important role to randomization in preventing bias, so that the absence of allocation concealment could overstate the intervention effect by 30–41 % [39]. In this study, since the funnel plot was not completely symmetrical, the bias would be overcome only with collection of more literatures. Thus, the clinicians must be aware of possible publication bias in the use of evidences to guide clinical practice, which might have a greater impact on the final conclusion. The majority of the studies analyzed focussed only on GISTs. However, some included studies had cases of other type gastric submucosal tumors (SMTs) such as neurilemmomas and leiomyoma. SMTs display a wide spectrum, ranging from benign to highly malignant, with GISTs being the most common [16, 40], and preoperative histologic diagnosis remains difficult. Because the sample size of remaining studies is still small for definitive conclusions on the safety and effectiveness of LAP and the larger the number of patients in a meta-analysis, the greater its power to detect a possible treatment effect. Therefore, we did not exclude the study. Although, such a low number does not imply a significant bias, it still can lead to clinical heterogeneity. Also, the majority of cases in our study are in the past 3 years, which is short for the low risk GISTs to develop recurrence, and the follow-up will continue.
Conclusions
Laparoscopic resection for gastric GISTs is a safe and feasible procedure, which will not increase the risks of tumor relapse and metastasis. However, the lack of randomized trials or high-quality, nonrandomized prospective studies does not allow for firm conclusions to be drawn. Randomized controlled trials or prospective cohort studies, which avoid selection and experimenter bias and control for confounding factors are necessary to adequately evaluate the status of laparoscopic resection for gastric GISTs.
References
Miettinen M, Majidi M, Lasota J (2002) Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer (Oxford, England: 1990) 38(Suppl 5):S39–S51
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD (2010) NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compreh Cancer Netw (JNCCN). 8 Suppl 2: S1–S41; quiz S42-44
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Xu X, Chen K, Zhou W, Zhang R, Wang J, Wu D, Mou Y (2013) Laparoscopic transgastric resection of gastric submucosal tumors located near the esophagogastric junction. J Gastrointest Surg 17:1570–1575
Kang WM, Yu JC, Ma ZQ, Zhao ZR, Meng QB, Ye X (2013) Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors. World J Gastroenterol WJG 19:5720–5726
Valle M, Federici O, Carboni F, Carpano S, Benedetti M, Garofalo A (2014) Gastrointestinal stromal tumors of the stomach: the role of laparoscopic resection. Single-centre experience of 38 cases. Surg Endosc 28:1040–1047
Lee CH, Hyun MH, Kwon YJ, Cho SI, Park SS (2012) Deciding laparoscopic approaches for wedge resection in gastric submucosal tumors: a suggestive flow chart using three major determinants. J Am Coll Surg 215:831–840
Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP (2007) Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg 204:356–364
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 327:557–560
Basu S, Balaji S, Bennett DH, Davies N (2007) Gastrointestinal stromal tumors (GIST) and laparoscopic resection. Surg Endosc 21:1685–1689
Chen YH, Liu KH, Yeh CN, Hsu JT, Liu YY, Tsai CY, Chiu CT, Jan YY, Yeh TS (2012) Laparoscopic resection of gastrointestinal stromal tumors: safe, efficient, and comparable oncologic outcomes. J Laparoendosc Adv Surg Tech A 22:758–763
Fisher SB, Kim SC, Kooby DA, Cardona K, Russell MC, Delman KA, Staley CA 3rd, Maithel SK (2013) Gastrointestinal stromal tumors: a single institution experience of 176 surgical patients. Am Surg 79:657–665
Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, Kubota T, Mukai M, Kameyama K, Sugino Y, Kumai K, Kitajima M (2006) Operative indications for relatively small (2–5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery 139:484–492
Wu JM, Yang CY, Wang MY, Wu MH, Lin MT (2010) Gasless laparoscopy-assisted versus open resection for gastrointestinal stromal tumors of the upper stomach: preliminary results. J Laparoendosc Adv Surg Techn Part A 20:725–729
Shimizu S, Noshiro H, Nagai E, Uchiyama A, Mizumoto K, Tanaka M (2002) Laparoscopic wedge resection of gastric submucosal tumors. Digest Surg 19:169–173
Matthews BD, Walsh RM, Kercher KW, Sing RF, Pratt BL, Answini GA, Heniford BT (2002) Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc 16:803–807
Ishikawa K, Inomata M, Etoh T, Shiromizu A, Shiraishi N, Arita T, Kitano S (2006) Long-term outcome of laparoscopic wedge resection for gastric submucosal tumor compared with open wedge resection. Surg Laparosc Endosc percutan Tech 16:82–85
Mochizuki Y, Kodera Y, Fujiwara M, Ito S, Yamamura Y, Sawaki A, Yamao K, Kato T (2006) Laparoscopic wedge resection for gastrointestinal stromal tumors of the stomach: initial experience. Surg Today 36:341–347
Nishimura J, Nakajima K, Omori T, Takahashi T, Nishitani A, Ito T, Nishida T (2007) Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs. open resection. Surg Endosc 21:875–878
Pitsinis V, Khan AZ, Cranshaw I, Allum WH (2007) Single center experience of laparoscopic vs. open resection for gastrointestinal stromal tumors of the stomach. Hepatogastroenterology 54:606–608
Catena F, Di Battista M, Fusaroli P, Ansaloni L, Di Scioscio V, Santini D, Pantaleo M, Biasco G, Caletti G, Pinna A (2008) Laparoscopic treatment of gastric GIST: report of 21 cases and literature’s review. J Gastrointest Surg 12:561–568
Silberhumer GR, Hufschmid M, Wrba F, Gyoeri G, Schoppmann S, Tribl B, Wenzl E, Prager G, Laengle F, Zacherl J (2009) Surgery for gastrointestinal stromal tumors of the stomach. J Gastrointest Surg 13:1213–1219
Goh BK, Chow PK, Chok AY, Chan WH, Chung YF, Ong HS, Wong WK (2010) Impact of the introduction of laparoscopic wedge resection as a surgical option for suspected small/medium-sized gastrointestinal stromal tumors of the stomach on perioperative and oncologic outcomes. World J Surg 34:1847–1852
Karakousis GC, Singer S, Zheng J, Gonen M, Coit D, DeMatteo RP, Strong VE (2011) Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol 18:1599–1605
Dai QQ, Ye ZY, Zhang W, Lv ZY, Shao QS, Sun YS, Tao HQ (2011) [Laparoscopic versus open wedge resection for gastrointestinal stromal tumors of the stomach: a clinical controlled study]. Zhonghua wei chang wai ke za zhi =. Chin J Gastrointest Surg 14:603–605
De Vogelaere K, Hoorens A, Haentjens P, Delvaux G (2013) Laparoscopic versus open resection of gastrointestinal stromal tumors of the stomach. Surg Endosc 27:1546–1554
Melstrom LG, Phillips JD, Bentrem DJ, Wayne JD (2012) Laparoscopic versus open resection of gastric gastrointestinal stromal tumors. Am J Clin Oncol 35:451–454
Lee HH, Hur H, Jung H, Park CH, Jeon HM, Song KY (2011) Laparoscopic wedge resection for gastric submucosal tumors: a size-location matched case-control study. J Am Coll Surg 212:195–199
Wan P, Yan C, Li C, Yan M, Zhu ZG (2012) Choices of surgical approaches for gastrointestinal stromal tumors of the stomach: laparoscopic versus open resection. Digest Surg 29:243–250
Pucci MJ, Berger AC, Lim PW, Chojnacki KA, Rosato EL, Palazzo F (2012) Laparoscopic approaches to gastric gastrointestinal stromal tumors: an institutional review of 57 cases. Surg Endosc 26:3509–3514
Kim KH, Kim MC, Jung GJ, Kim SJ, Jang JS, Kwon HC (2012) Long term survival results for gastric GIST: is laparoscopic surgery for large gastric GIST feasible? World J Surg Oncol 10:230
Shu ZB, Sun LB, Li JP, Li YC, Ding DY (2013) Laparoscopic versus open resection of gastric gastrointestinal stromal tumors. Chin J Cancer Res Chung-kuo yen cheng yen chiu 25:175–182
Lee PC, Lai PS, Yang CY, Chen CN, Lai IR, Lin MT (2013) A gasless laparoscopic technique of wide excision for gastric gastrointestinal stromal tumor versus open method. World J Surg Oncol 11:44
Chen K, Xu XW, Zhang RC, Pan Y, Wu D, Mou YP (2013) Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol WJG 19:5365–5376
Law WL, Lee YM, Choi HK, Seto CL, Ho JW (2007) Impact of laparoscopic resection for colorectal cancer on operative outcomes and survival. Ann Surg 245:1–7
Chen K, Mou YP, Xu XW, Cai JQ, Wu D, Pan Y, Zhang RC (2014) Short-term surgical and long-term survival outcomes after laparoscopic distal gastrectomy with D2 lymphadenectomy for gastric cancer. BMC Gastroenterol 14:41
Ephgrave KS, Kleiman-Wexler R, Pfaller M, Booth B, Werkmeister L, Young S (1993) Postoperative pneumonia: a prospective study of risk factors and morbidity. Surgery 114:815–819; discussion 819-821
Schulz KF, Chalmers I, Hayes RJ, Altman DG (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. J Am Med Assoc JAMA 273:408–412
Walsh RM, Heniford BT (2001) Laparoendoscopic treatment of gastric stromal tumors. Semin Laparosc Surg 8:189–194
Acknowledgments
This study was supported by Zhejiang Key Subject of Medical Science Foundation (grant No.11-CX-21).
Disclosures
Ke Chen, Yu-Cheng Zhou, Yi-Ping Mou, Xiao-Wu Xu, Wei-Wei Jin and Harsha Ajoodhea have no conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, K., Zhou, YC., Mou, YP. et al. Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg Endosc 29, 355–367 (2015). https://doi.org/10.1007/s00464-014-3676-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3676-6