Abstract
Aim
To review management, outcome and the lessons learnt from a laparoscopic approach to GISTs.
Method
All cases of GIST presenting to the upper GI MDT between 2000 and 2006 were reviewed. Presentation, preoperative investigations, management and follow-up were recorded. Surgical resection using a laparoscopic approach, where feasible was the preferred management.
Results
25 consecutive patients that included one oesophageal, three oesophago-gastric, 19 gastric and two smallbowel GISTs were treated between 2000 and 2006. There were 11 male and 14 females with a median age of 68 (25–90) years. Clinical presentation was: gastrointestinal bleed 15, pain 6, dysphagia 2, anaemia 3, weight loss 1, and asymptomatic 2. Out of 25, four were inoperable and treated with imatinib. 17 laparoscopic (including 2 conversions) and four open procedures were performed. Two (both GISTs close to the oesophago-gastric junction) required reoperation due to surgical-related morbidity. Of the 25, five were high-, 11 intermediate- and nine low-risk GISTs. No recurrences in follow-up (median 24, range 6–75) months was observed.
Conclusion
GISTs can safely and effectively be treated laparoscopically although larger GISTs in difficult anatomical locations may require open surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastrointestinal stromal tumors (GISTs) are rare tumors and represent 0.1–3% of all gastrointestinal cancers with an estimated incidence of 15/million [1–3]. There are approximately 900 new cases a year in the UK. The term GIST was coined in 1983 [4]. The tumor originates from the interstitial cells of Cajal and are characterised by the overexpression of the tyrosin kinase receptor KIT [5]. The stomach (60%) has been identified as the most common site for GISTs followed by 15% each within the small and large bowel [6–10]. Surgery provides the most effective treatment for resectable GISTs [11]. Several retrospective analyses report resection rates of 70–86% [12]. Lymph node metastases are rare and localised resection with a clear margin of 1 to 2 cm appears to be an adequate treatment [13, 14]. Recent evidence has shown that survival is dependent on tumor size and histological features rather than the extent of resection [6]. Many GISTs can therefore be treated without major anatomical resections [15] and are suitable for minimally invasive surgery; this is especially true for gastric GISTs [10, 11, 16–19]. The aim of this study was to review presentation, management, follow up and lessons that were learnt from the laparoscopic approach to gastrointestinal stromal tumors presenting to the upper GI unit over the last six years.
Materials and methods
The operative and histopathological database was reviewed to identify all GISTs presenting to the upper gastrointestinal multidisciplinary meetings from 2000 to 2006. Patient demographics, clinical presentation and anatomical location of the tumors were analyzed. Perioperative parameters measured included operative timings, postoperative recovery including morbidity, mortality and length of hospitalisation. Tumor histopathologic characteristics including size, presence of necrosis and ulceration, immunohistochemistry, and mitotic activity were also reviewed.
After initial diagnoses, computerised tomography and endoscopic ultrasonography were performed to complete the staging. Where anatomically and oncologically feasible, the intent was to treat patients laparoscopically. Those considered unsuitable for resection were discussed in the multidisciplinary meetings and started on imatinib therapy.
Postoperatively, nasogastric tubes were used routinely for GISTs of the foregut. A nonionic swallow was performed for the cases involving the oesophagus and oesophago-gastric junction. Patients were discharged following tolerating a regular diet.
Patients were routinely followed up in the clinic initially on a three-monthly basis for the first two years, every six months for the next two years and then yearly. An oesophagogastroduodenoscopy and computerised tomography was obtained yearly.
Data are expressed in percentage and median.
Results
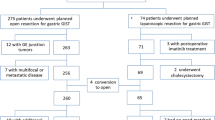
Twenty five consecutive patients with gastrointestinal stromal tumor were treated between 2000 and 2006. There were 11 men and 14 women with a median age of 68 years. The primary presenting symptoms for our patients are summarised in Table 1, with the gastrointestinal bleed being the commonest noted in 15 (60%). The stomach was the commonest site of GIST, identified in 19 (76%) patients.
Twenty five patients underwent preoperative oesophagogastroduodenoscopy and one capsular enteroscopy. In addition, for staging all 25 patients underwent abdominal computerised tomography (CT) scan and 11 (44%) also had an endoscopic ultrasound. One patient with gastric GIST had an incidental left renal cell carcinoma.
Of the 25, 21 (84%) patients had successful surgical resections (Table 2). Four (16%) were inoperable at presentation, all were GISTs in the stomach. Three had liver metastases and another patient was suffering from a diffuse B cell lymphoma. This group were treated with imatinib and had a median survival of 29 (3–54) months.
Of the 21, 17 had laparoscopic (including two conversions) and four had open procedures (Table 2). One patient with oesophago-gastric GIST had laparoscopic converted to an open procedure due to its large size (8 cm) and difficult assess. Similarly, another patient with jejunal GIST had laparoscopic converted to an open procedure due once again to difficult access. Of the 15 gastric GISTs, 12 underwent laparoscopic and three had open operations. The median operating time, oral diet and hospital stay for the 15 patients undergoing laparoscopic GIST resections were 90 mins, 5 and 7 days respectively. Two patients with oesophago-gastric GISTs had major morbidity with anastomotic leaks from staple lines. One following laparoscopic resection had a leak detected on a postoperative contrast study. He was reoperated and had a protracted postoperative period from chest complications. The second patient converted to an open procedure during his initial operation, had a clinical leak within 24 hours requiring a reoperation. His postoperative recovery was uneventful. The median operating time, oral diet and hospital stay for the six patients undergoing open (including the two conversions) resections were 135 mins, 7 and 12 days, respectively.
The median follow-up for our study was 24 (6–75) months. Of the 21 postresection patients, 14 (67%) have undergone oesophagogastroduodenoscopy. Seventeen (81%) of the 21 patients have no signs of recurrence in their follow-up CT scan. The other four are awaiting their first one-year follow up investigations.
Histopathology and immunohistochemistry (Table 3)
The median tumor size for this study was 6 cm (2–11cm) and the median tumor size resected laparoscopically and open was 5.5 (2–11) cm and 7.5(3–10) cm respectively. Of the 21 resections, mucosal ulceration was noted in four (19%) and tumor necrosis in seven (33%) patients. All lesions had negative resection margins.
Following the GIST risk criteria [20], of the 25 patients, five were high-risk, 11 intermediate-risk and nine low-risk patients (Table 3). The high-risk oesophago-gastric GIST had laparoscopic converted to an open procedure. Of the four high-risk stomach GISTs, three were treated medically with imatinib due to metastases and subsequently died and the fourth one had a successful laparoscopic resection.
The single oesophageal and two oesophago-gastric intermediate-risk GISTs were treated through open and laparoscopic procedures respectively. Of the eight intermediate-risk stomach GISTs, five had laparoscopic resections and three had open procedures.
Of the seven low-risk stomach GISTs, six had laparoscopic resections. The one low-risk stomach GIST treated medically with imatinib due to co-existent diffuse B cell lymphoma died after three months. Of the two low-risk jejunal GISTs, one had laparoscopic and the other had laparoscopic converted to an open procedure.
Discussion
Gastrointestinal stromal tumors (GISTs), although rare, are the most common mesenchymal tumors of the gastrointestinal tract. GISTs predominantly occur in older Caucasian adults with no significant sex difference [6, 11]. Presentation depends on size and location of the tumor [20]. Gastrointestinal bleeding (50%) is the most common presentation, followed by abdominal pain (20–50%) and obstruction (20%) and approximately a third are detected incidentally [1].
Surgery is the preferred management for GISTs where feasible. There is still debate regarding the most appropriate operative approach and the extent of resection required. The aim of surgery is the complete removal of the tumor with negative resection margins [6, 9] and where ever possible with the preservation of anatomical function. Lymph node metastases are very rare and routine lymphadenectomy is not required [6, 21]. Wedge resection of gastric GISTs has been widely reported to be successful [3, 7, 9, 10, 22, 23]. There is also evidence that laparoscopic resection of GISTs is effective [24–27] with minimal morbidity and no reported mortality [15]. If a laparoscopic approach is contemplated, several factors including patient characteristics, tumor size, location, invasion as well as the surgeon’s experience and expertise need to be considered [15]. The aim of laparoscopic surgery should be complete removal of the tumor with clear resection margins. Tumor rupture during laparoscopy should be avoided, as peritoneal seeding affects disease free period and overall survival [28]. Tumor size and location may warrant more extensive surgery and an open approach may be preferred [29, 30].
In this study of 25 patients, 21 were deemed suitable for surgical resection. 15 (60%) had successful laparoscopic resections (two oesophago-gastric junction, 12 stomach and one jejunum). Two GISTs located each in the oesophago-gastric junction and jejunum had laparoscopic converted to open procedures because of their difficult access and tumor size. Three (one oesophagus and two stomach) other GISTs had planned open procedures, once again because of their size and location, and the fourth underwent simultaneous left radical nephrectomy.
Resection margins were clear in all cases, confirming the oncological safety of laparoscopic approach. Evidence suggests that tumor size singularly is not a contraindication to the laparoscopic approach [15] is also supported by our laparoscopic removal of an 11 cm gastric GIST. Increasing surgical expertise with laparoscopic upper gastrointestinal surgery together with advancement in laparoscopic instrumentation has made laparoscopic approach to GISTs all the more appealing. The combination of a large tumor and difficult location (oesophagus/oesophago-gastric junction/proximal stomach) can make laparoscopic approach all the more challenging. In this study, those patients converted to an open procedure or having an open procedure initially (one oesophageal, one oesophago-gastric junction, two proximal stomach GISTs, and one jejunal) had a median tumor size of 9 cm. The two patients requiring reoperation due to the staple line leakage had GISTs located in the oesophago-gastric junction. Large GISTs around the oesophago-gastric junction may be a challenge laparoscopically and an open approach should be considered.
GISTs have an increasing malignant potential based on tumor size and mitotic count. These parameters should be used to assess prognosis in all cases [20, 31, 32]. The risk of disease recurrence increases on the basis of size >5 cm and mitotic number >5/HPF from low through intermediate to high [20, 33]. Most recurrences occur within the first two years following surgery [7] and follow-up should be stratified according to the risk [34]. Median survival for metastatic/irresectable GISTs ranges from 6–18 months approximately [35]. Chemotherapy and radiotherapy are ineffective [2]. Imatinib (glivec) is currently the only approved treatment for KIT-positive malignant metastatic or unresectable GISTs [33]. Four patients receiving imatinib in our study had a median survival of 29 months.
GISTs have an unpredictable behavior and even with risk stratification careful follow up is required. Even though the majority of those GISTs likely to recur after surgery will do so within two years, follow-up beyond this period would seem sensible. A recent population-based study of intermediate-risk primary GISTs (<5cm and 6–10/5 0HPFs or 5–10 cm and <5/50 HCF) treated surgically found no tumor recurrences or metastases, no tumor related death and no differences in survival compared with the age- and sex- matched general population [36]. There is also evidence of patients with localised disease have a 93% five-year survival [29]. In this study, 95% of the operated patients were in low or intermediate-risk GIST group and there have been no recurrences with a median follow up of 24 months.
GISTs of the fore- and midgut can safely and effectively be treated laparoscopically with good results, although larger GISTs in difficult anatomical locations should preferably be approached through open surgery.
References
Rossi CR, Mocellin S, Mancarelli R, Foletto M, Pilati P, Nitti D (2003) Gastrointestinal stromal tumours: from a surgical to a molecular approach. Int J Cancer 107:171–176
Kindblom LG (2003) Education Session E450, oral presentation “Gastrointestinal Stromal Tumours Diagnosis, Epidemiology and Prognosis” in Gastrointestinal Stromal Tumour: Current management and Future Challenges. Chair: Blanke CD. ASCO
Corless CL, Fletcher JA, Heinrich MC (2004) Biology of gastrointestinal stromal tumours. J Clin Oncol 22:3813–3825
Mazur MT, Clark HB (1983) Gastric stromal tumours. Reappraisal of histogenesis. Am J Surg Pathol 7:507–519
Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM (1998) Gastrointestinal stromal tumours show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 152:1259–1269
DeMatteo RP, Lewis JJ, Leung D, Jeung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumour: recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Nowain A, Bhakta H, Pais S, Kanel G, Verma S (2005) Gastrointestinal stromal tumours: clinical profile, pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol 20(6):818–824
Miettinen M, Virolainen M, Maarit Sarlomo R (2005) Gastrointestinal stromal tumour of the stomach: a clinicopathologic, immunohistochemical and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29:52–68
Heinrich MC, Corless CL (2005) Gastric GI stromal tumours (GISTs): the role of surgery in the era of targeted therapy. J Surg Oncol 90:195–207
Cheng HL, Lee WJ, Lai IR, Yuan RH, Yu SC (1999) Laparoscopic wedge resection of benign gastric tumour. Hepatogastroenterology 46:2100–2104
Neuhaus SJ, Clark MA, Hayes AJ, Thomas JM, Judson I (2005) Surgery for gastrointestinal stromal tumour in the post-imatinib era. ANZ J Surg 75:165–172
Roberts PJ, Eisenberg B (2002) Clinical presentation of gastrointestinal stromal tumours and treatment of operable disease. Eur J Cancer 38:S37–38
Mathews BD, Walsh RM, Kercher KW, Sing RF, Pratt BL, Answini GA, Heniford BT (2002) Laparoscopic vs open resection of gastric stromal tumours. Surg Endosc 16:803–807
Cuschieri A (2000) Laparoscopic gastric resection. Surg Clin North Am 80:1269–1284
Novitsky YW, Kercher KW, Sing RF, Heniford BT (2006) Long-term outcome of laparoscopic resection of gastric gastrointestinal stromal tumours. Ann Surg 243(6):738–747
Heniford BT, Arca MJ, Walsh RM (2000) The mini-laparoscopic intragastric resection of a gastroesophageal stromal tumour: a novel approach. Surg Laparosc Percutan Tech 10:82–85
Walsh RM, Ponsky J, Brody F, Mathews BD, Heniford BT (2003) Combined endoscopic/laparoscopic intragastric resection of gastric stromal tumours. J Gastrointest Surg 7:386–392
Geis WP, Baxt R, Kim HC (1996) Benign gastric Tumours: minimally invasive approach. Surg Endosc 10:407–410
Nguyen NT, Jim J, Nguyen A, Lee J, Chang K (2003) Laparoscopic resection of gastric stromal tumour: a tailored approach. Am Surg 69:946–950
Fletcher CD, Bermenn JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Renotti H, Rubin BP, Shmokler B, Solin LH, Weiss SW (2002) Diagnosis of gastrointestinal stromal tumours: a consensus approach. Hum Pathol 33:459–465
DeMatteo RP, Heinrich MC, El-Rifai WM, Demetri G (2002) Clinical management of gastrointestinal stromal tumours: before and after STI-571. Hum Pathol 33:466–477
Rosen MJ, Heniford BT (2005) Endoluminal gastric surgery: the modern era of minimally invasive surgery. Surg Clin North Am 85:989–1007
Yoshida M, Otani Y, Ohgami M, Kubota T, Kumai K, Mukai M, Kitajima M (1997) Surgical management of gastric leimyosarcoma: evaluation of the propriety of laparoscopic wedge resection. World J Surg 21:440–443
Ludwig K, Wilhelm L, Scharlau U, Amtsberg G, Bernhardt J (2001) Laparoscopic-endoscopic rendezvous resection of gastric tumours. Surg Endosc 16:1561–1565
Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H (2002) Laparoscopic intragastric stapled resection of gastric submucosal tumours located near the esophgaogastric junction. Surg Endosc 16:177–179
Rothlin M, Schob O (2001) Laparoscopic wedge resection for benign gastric tumour. Surg Endosc 15:893–895
Granger SR, Rollins MD, Mulvihill SJ, Glasgow RE (2006) Lessons learned from laparoscopic treatment of gastric and gastroesophageal junction stromal tumours. Surg Endosc 20:1299–1304
Ng EH, Pollock RE, Munsell ME, Atkinson EN, Romsdahl MM (1992) Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Ann Surg 215:68–77
Fujimoto Y, Nakanishi Y, Yoshimura K, Shimoda T (2003) Clinicopathologic study of primary malignant gastrointestinal stromal tumour of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer 6:39–48
Demetri GD, Blanke CD (2004) NCCN Task Force Report. Optimal management of patients with gastrointestinal stromal tumours (GIST): expansion and update of NCCN Clinical Guidelines. J Natl Comp Cancer Network 2(suppl):1–26
Miettinen M, El-Rifal W, Sobin LH, Lasota J (2002) Evaluation of malignancy and prognosis of gastrointestinal stromal tumours: A review. Hum Pathol 33:478–483
Yan H, Marchettini P, Acherman YI, Gething SA, Brun E, Sugarbaker PH (2003) Prognostic assessment of gastrointestinal stromal tumour. Am J Clin Oncol 26:221–228
Eisenberg BL, Judson I (2004) Surgery and imatinib in the management of GIST: emerging approaches to adjuvant and Neoadjuvant therapy. Ann Surg Oncol 11(5):465–475
Reichardt P (2003) Practical aspects of managing gastrointestinal stromal tumours. Monographs in Gastrointestinal Stromal Tumours 1:3–8
Blanke CD, Eisenberg BL, Heinrich MC (2001) Gastrointestinal stromal tumours. Curr Treat Options Oncol 2:485–491
Buemming P, Meis-Kindblom JM, Kindblom LG, Gustavsson B, Thakrar B, Engstrom K, Ahlman H, Nilsson BE (2003) Is there an indication for adjuvant treatment with imatinib mesylate in patients with aggressive gastrointestinal stromal tumours (GISTs)? Proc Am Soc Clin Oncol 22:818
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basu, S., Balaji, S., Bennett, D.H. et al. Gastrointestinal stromal tumors (GIST) and laparoscopic resection. Surg Endosc 21, 1685–1689 (2007). https://doi.org/10.1007/s00464-007-9445-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-007-9445-z