Abstract
Background
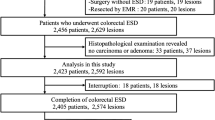
Endoscopic submucosal dissection (ESD) for colorectal tumors is technically difficult due to the anatomy of the large intestine, with its narrow lumen, thin walls, and redundancy. Here, we assessed factors associated with incomplete resection and difficult colorectal ESD.
Methods
Between November 2009 and April 2013, we performed ESD on 151 consecutive colorectal tumors in 147 patients. We evaluated the clinical outcomes of all cases and conducted multiple logistic regression analysis of the following factors related to incomplete resection and difficult procedure: age, gender, location (right colon, left colon or rectum), tumor size (diameter ≥40 or <40 mm), operation time, morphology [granular-type laterally spreading tumor (LST-G), non-granular-type laterally spreading tumor (LST-NG), or protruded type], fibrosis, and paradoxical movement during the procedure. A procedure that required more than 120 min was defined as a difficult colorectal ESD.
Results
Average tumor size was 32.1 ± 10.7 mm, and the average procedure length was 71.8 ± 49.5 min. The rate of en bloc resection was 94.7 %, while that of en bloc curative resection was 86.8 %. Perforation occurred in 1.3 % of the ESD procedures. Multivariate logistic regression analysis revealed that only severe fibrosis [odds ratio (OR) 4.51; 95 % confidence interval (CI) 1.36–14.91, p = 0.014] contributed to incomplete resection and that a tumor size exceeding 40 mm (OR 5.73 [95 % CI 1.66–19.74], p = 0.006), severe fibrosis (OR 23.31 [95 % CI 6.59–82.54], p < 0.001), and paradoxical movement (OR 4.26 [95 % CI 1.11–16.44], p = 0.035) were independent factors exacerbating the difficulty of colorectal ESD.
Conclusions
Severe fibrosis contributed to both incomplete resection and difficult colorectal ESD. Larger tumor size and paradoxical movement during the procedure were independent factors contributing to the difficulty of colorectal ESD. These factors might enable endoscopists to develop strategies for treating colorectal ESD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endoscopic submucosal dissection (ESD) is more feasible than conventional endoscopic mucosal resection (EMR) for epithelial neoplasia in the esophagus, stomach, and colorectum via en bloc resection to facilitate accurate pathological diagnosis and reduce the risk of recurrence as assessed through long-term follow-up [1–3]. Although ESD tends to result in better clinical outcomes than EMR, it requires a high degree of training and technical skill, particularly for colorectal lesions compared with those of gastric lesions [4–6]. Due to the anatomical characteristics of the colon, such as a thin wall, sparse muscle layer, and tortuous structure, colorectal ESD also carries a high risk of perforation and has a longer procedure time compared to conventional EMR [4, 6–8].
Perforation during colorectal ESD occurs due to a number of factors, including the presence of fibrosis and large-sized lesions, location in the colon, and endoscopists having relatively little experience [8–10]. Fibrosis in particular is one of the major contributors to perforation during colorectal ESD [4, 6], and causes an increase in the number of incomplete resections [4, 6, 11, 12]. In addition, paradoxical movement of the colonoscope during ESD, possibly caused by air accumulation in the abdomen due to lengthy procedure time [12–14], can cause coagulation of the muscularis propria, which may also result in perforation.
Here, in order to assess the factors of incomplete resection and difficult colorectal ESD, we retrospectively analyzed factors, such as fibrosis and paradoxical movement, which might be associated with cases of incomplete resection and lengthy procedure time.
Materials and methods
Patients
We reviewed the records of 151 lesions in 147 consecutive patients with colorectal epithelial neoplasms at the Toho University Ohashi Medical Center endoscopy unit between November 2009 and April 2013. This retrospective study was approved by the Toho University Ohashi Medical Center review board (Institutional review board numbers: 19, 22-23 and 13-74). Written informed consent was obtained from all subjects.
The indication criteria for colorectal ESD were as follows: lesions exceeding 20 mm in size, lesions with fibrosis caused by previous endoscopic treatment or biopsies, local residual or recurrent intramucosal lesions that showed a non-lifting sign after EMR, and invasive carcinomas with slight submucosal penetration [submucosal invasive carcinoma (SM1) or <1,000 μm below the muscularis mucosa] [4, 8, 9, 15].
The endoscopic appearance of the tumors was classified according to the Paris endoscopic classification [16]. Tumors were macroscopically classified as protruding large tumors (0-I) or one of four subtypes of laterally spreading tumors (LSTs). LSTs were first divided into granular-type laterally spreading tumors (LST-Gs) or non-granular-type laterally spreading tumors (LST-NGs). LST-Gs were then subdivided into homogenous-type and nodular-mixed-type tumors and LST-NGs into flat-elevated-type and pseudo-depressed-type tumors [9, 15–17].
We excluded patients with lesions diagnosed to be submucosal deep invasion cancers using magnification chromoendoscopy in preoperative examination [8, 18]. We also excluded patients with a submucosal tumor (SMT) or inflammatory bowel disease and local residual or recurrent lesions after EMR, as there was fibrosis beneath these lesions after EMR. Tumor locations were grouped into the right colon (cecum, ascending colon, and transverse colon), left colon (descending colon and sigmoid colon), and rectum [4, 19]. Histopathological diagnosis was made according to the World Health Organization classification.
En bloc resection was considered when the tumor was resected as a single piece and complete en block resection was ascribed when the tumor was removed en bloc with tumor-free lateral and basal margins (R0) [4, 6, 9]. Incomplete resection constituted tumors that were removed in multiple segments, or when lateral or basal margins were positive for tumor invasion, or when the margins could not be evaluated due to artificial burn effects or insufficient reconstruction of piecemeal fragments [9].
Curative resection was deemed when an R0 resection was performed and submucosal invasion <1,000 μm from the muscularis mucosae, lymphatic invasion, vascular involvement, budding, and poorly differentiated components was absent [5, 7, 20].
Additional surgery was recommended when the tumor was diagnosed as invasive carcinoma with deep submucosal invasion (>1,000 μm below the muscularis mucosae) or exhibited risk factors for lymph node metastasis such as lymphatic invasion, vascular involvement, budding, or poor differentiation [ 4, 18, 21 ].
ESD procedure
All patients received a low-fiber diet before the ESD and underwent bowel preparation with mosapride citrate (Dainippon Sumitomo Pharma Co. Ltd., Osaka, Japan) and 2 L of polyethylene glycol-electrolyte lavage solution (Ajinomoto Pharmacy, Tokyo, Japan) on the morning of examination.
Colorectal ESD was performed in accordance with the previous reports via a modified protocol by a single endoscopist who had performed more than 200 cases of gastric ESD and 50 cases of colorectal ESD [15, 22]. ESD was performed using a water jet endoscope (PCF-Q260JI, PCF-Q260AZI and GIF-Q260J; Olympus Medical Systems Co., Tokyo, Japan) with a transparent hood attached to the tip. A CO2 insufflation system was used to reduce any discomfort experienced by the patient throughout the procedure and to promote the absorption of leaked gas from perforation.
A dual knife was used for the majority of cases and either or both a hook knife (Olympus KD-620LR) or a SB Knife Jr. (Sumitomo Bakelite, Tokyo, Japan) for situations in which dissection might cause muscular injury [21, 23]. After injection of Glyceol® (Chugai Pharmaceutical Co., Tokyo, Japan) (10 % glycerol and 5 % fructose in normal saline solution) and sodium hyaluronate into the submucosa [22, 24], an initial incision was performed with a dual knife.
A high-frequency generator (ICC200; ERBE Elektromedizin GmbH, Tubingen, Germany) was used during incision of the mucosa, set in the endocut mode, effect 3 (80 W), and for the submucosal dissection with the forced coagulation mode (40 W). Hemostatic forceps (Coagrasper, FD-411QR; Olympus, Tokyo, Japan) were used in the soft coagulation mode (50 W) to achieve hemostasis during ESD or prevent possible bleeding from visible vessel in the artificial ulcer immediately after the procedure.
Patients were sedated with pethidine hydrochloride (Opistan®; Tanabe Pharmaceutical Co., Tokyo, Japan) at a single intravenous dose of 0.6 mg/kg before the procedure (conscious sedation), and cardiopulmonary function was monitored throughout the procedure. If the patient experienced abdominal discomfort and requested further sedation during the procedure, midazolam (Astellas Pharma Inc., Tokyo, Japan) was intravenously administered. To prevent colonic wall spasms, scopolamine butyl bromide or glucagon was intravenously administered before colonoscopy or during the procedure as necessary.
Factors associated with difficult colorectal ESD procedures
Difficult cases of colorectal ESD were defined as those requiring more than 120 min to complete the procedure. Factors of difficult colorectal ESD were as follows: patient factors (age, gender), tumor-related factors (size, location, morphology, presence of fibrosis), and procedure-related factors (procedure time, paradoxical movement of colonoscopy).
Paradoxical movement was defined as the tip of the colonoscope retracted even when the endoscope advanced outside the patient because axial force exerted by the endoscopist was not translated to forward motion of the tip of the colonoscope due to looping [25].
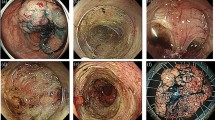
Fibrosis was classified into three groups (F0: no fibrosis, F1: mild fibrosis, F2: severe fibrosis) based on endoscopic findings obtained at the time of injection of sodium hyaluronate with indigo carmine (Fig. 1) [11]. Severe fibrosis was defined as the presence of a submucosal layer with a white muscular-like structure without a blue transparent layer following the injection of a mixture of sodium hyaluronate and indigo carmine into the submucosal layer [11].
Classification of fibrosis was divided into three groups (F0–F2) according to endoscopic findings. A F0: no fibrosis, appears as a blue transparent layer. B F1: mild fibrosis, emerges as a white, web-like structure in the blue submucosal layer. C F2: severe fibrosis, with submucosal layer appearing as a white, muscle-like structure without a blue transparent layer (Color figure online)
Complications
Perforation during an ESD was defined as immediate perforation which was deemed as a full-thickness defect during the ESD. Delayed perforation was defined as any perforation occurring after completion of the procedure [7]. Postoperative bleeding was defined as clinical evidence of bleeding manifested by hematochezia within 14 days after the procedure that required endoscopic hemostasis [17].
Statistical analysis
Continuous variables were tested for normality and analyzed using the unpaired t test. Categorical variables were analyzed using the Chi squared test or Fisher’s exact test. Intention-to-treat analysis was used for all comparisons after the beginning of the examination. Univariate and forward stepwise multivariate logistic regression analyses were performed to assess the factors of incomplete resection and difficult ESD (>120 min.). Odds ratios (ORs) and 95 % confidence intervals (95 % CIs) were calculated to evaluate the predictors of difficult ESD. All analyses were conducted using SPSS for Windows, version 19 (SPSS Inc., Chicago, IL, USA).
Results
A total of 151 lesions with 147 patients were treated with colorectal ESD between November 2009 and April 2013 at the Toho University Ohashi Medical Center. Demographic and clinical data are shown in Table 1. The mean age of the patients was 71.6 ± 11.7 (range, 37–89) years. The female to male ratio was 41:59. The average tumor size was 32.1 ± 10.7 (range, 20–85) mm. Lesions were mainly located in the rectum (19.9 %), sigmoid colon (18.5 %), transverse colon (22.5 %), and ascending colon (22.5 %). LST-G and LST-NG were near equally distributed in the colon (LST-G, 49 %; LST-NG, 45 %).
Histopathological assessment
The most frequent histological type of resected lesion was intramucosal adenocarcinoma (80/151, 53 %). The second most common histological type was high-grade adenoma (40/151, 26.5 %). Eight (5.3 %) cases of superficial submucosal cancer and seven (4.6 %) cases of deep submucosal cancer were noted (Table 1). One (0.7 %) case of deep submucosal invasion was poorly differentiated small-cell neuroendocrine carcinoma [26].
Resectability and procedure-related outcomes
A summary of resectability and other outcomes is shown in Table 2. En bloc resection was achieved in 143 of the 151 treated lesions (94.7 %). While en bloc R0 curative resection was achieved in 86.8 % of cases, incomplete resection was only achieved in 13.2 % (20 cases) of resected lesions (Table 2). The reasons for incomplete resection were as follows: piecemeal, three cases (2.0 %); R1 (lateral), two cases (1.3 %); R1 (basal), seven cases (4.6 %); and Rx, eight cases (5.3 %). Of the 20 cases of incomplete resection, 7 carcinomas with either or both deep submucosal invasion or lymphovascular infiltration and 2 shallow invasive carcinomas with lymphatic invasion were considered to be at risk of lymph node metastasis. Of the nine cases requiring additional surgery, eight underwent additional resection accompanied by lymph node dissection. The one remaining case was followed up, as the patient refused surgical intervention. Of the eight cases that underwent additional surgery, only one with poorly differentiated neuroendocrine carcinoma had metastasis to the lymph nodes, with the remaining seven cases exhibiting no lymph node metastasis. Univariate analysis showed that longer procedure time (≥120 min) (p < 0.001) and severe fibrosis (p < 0.001) were factors significantly associated with incomplete resection. A trend for incomplete resection in the group which experienced paradoxical movement during the procedure was also noted (p = 0.05) (Table 3). Multivariate logistic regression analysis revealed that severe fibrosis (OR 4.51 [95 % CI 1.36–14.91], p = 0.014) was the only independent factor related to incomplete resection. Procedures that required more than 120 min tended to involve incomplete resection (OR 3.03 [95 % CI 0.83–11.07], p = 0.094). The average procedure time was 71.8 ± 49.5 (range, 15–340) min. Severe fibrosis occurred in 27 cases (17.9 %) and paradoxical movement during the procedure in 15 cases (9.9 %) (Table 2).
Perforation occurred in two cases (1.3 %). For one patient, the defect was closed using endoscopic clipping and for another patient abdominal radiograph revealed perforation after the procedure. Each patient was treated by conservative management without operation. We noted no cases of delayed perforation and two cases of delayed bleeding, both of which were treated conservatively after homeostasis with endoclips. No cases of bleeding or perforation required blood transfusion or surgical intervention.
Factors associated with difficult colorectal ESD
Univariate analysis showed that tumor size (≥40 mm, p < 0.001), severe fibrosis (p < 0.001), and paradoxical movement during the procedure (p = 0.005) were significantly associated with difficult colorectal ESD (Table 4). Multivariate logistic regression analysis revealed that tumor size exceeding 40 mm (OR 5.73 [95 % CI 1.66–19.74], p = 0.006), severe fibrosis (OR 23.31 [95 % CI 6.59–82.54], p < 0.001), and paradoxical movement (OR 4.26 [95 % CI 1.11–16.44], p = 0.035) were three independent factors related to difficult procedure.
Discussion
In the present study, we confirmed that colorectal ESD achieved a high rate of en bloc resection and that severe fibrosis was a factor in both incomplete resection and difficult colorectal ESD. We also found that a larger tumor size and paradoxical movement during the procedure contributed to difficult colorectal ESD and that lengthy procedure time might result in incomplete resection. The en bloc resection rate was 94.7 %, and en bloc curative resection rate was 86.8 %, findings consistent with recent studies (86.8–98.6 %; 79.8–95.6 %, respectively) [1, 2, 4, 8, 10, 18, 21, 27, 28].
Previous reports have stated that, in the presence of fibrosis, a tumor located on the right side or with a large size can prevent complete resection [4, 11]. In the present study, multivariate logistic regression analysis revealed that severe fibrosis and prolonged procedure time (>120 min) were associated with incomplete resection, which included R1 and Rx resection.
In cases of severe fibrosis, injected hyaluronic acid or Glyceol® leaks rapidly into submucosa, and a blue transparent layer in the submucosa is not observed [11, 23]. As a result, when elevation of submucosa by injected fluid is poor or not observed, determining the orientation of the tumor to be dissected can prove difficult for endoscopists, thereby affecting completion of the colorectal ESD [13]. Matsumoto et al. [11] reported that, in cases of lesions with severe fibrosis, the complete en bloc resection rate was low and the perforation rate was high. The rate of complete en bloc resection for lesions with fibrosis in our present study was consistent with that in previous reports [4, 11], but severe fibrosis did not significantly increase the perforation rate in our study.
The perforation rate was relatively low (1.3 %) in our study compared to previous reports [4–6, 8, 27], and we noted no cases of delayed perforation. One possible reason for the low perforation rate is that the endoscopist made the incision in the upper-part of the submucosal layer for lesions with severe fibrosis, as the rate of cutting into the specimen in lesions with severe fibrosis was higher than in those without (3/27 with [11.1 %] vs. 2/124 without [1.6 %]: p = 0.04). Another reason for this relatively low rate of perforation is that recent improvements in endoscopic devices and the combination use of ancillary devices have reduced complications such as perforation in ESD [7, 23]. In the present study, a hook knife was used in a higher number of cases of severe fibrosis than of non-fibrosis or moderate fibrosis (14/27 [51.9 %] vs. 36/124 [29 %]: p = 0.022), and an SB knife Jr was used as an ancillary device in seven cases of severe fibrosis. Using these ancillary devices for severe fibrosis might prevent perforation.
Long procedure time is the one of the limitations of colorectal ESD compared to conventional endoscopic resection (CER) [7]. We defined a procedure time of more than 120 min as difficult. Multivariate logistic regression analysis revealed that tumor size (>40 mm), severe fibrosis, and paradoxical movement were factors associated with the difficult colorectal ESD. Nakajima et al. [7] reported that the average procedure time for colorectal lesions measuring more than 40 mm required more than 120 min, findings which coincided with our own. Colorectal ESD of large-sized lesions is considered to be a difficult and time-consuming procedure compared with conventional EMR and should, therefore, be performed by experienced endoscopists [7, 8].
Fibrosis is one of the factors associated with difficult ESD [12]. Severe fibrosis tended to appear more frequently in lesions measuring more than 40 mm in diameter than those smaller in size (11/39 [%] vs. 16/112 [%]: p = 0.051). Inada et al. [12] also reported a significantly higher incidence of severe fibrosis in lesions larger than 40 mm among protruding tumors. Matsumoto et al. [11] reported that the incidence of severe fibrosis was higher in LST-GM than in other morphologic types of LSTs. In our study, however, we observed no significant difference in the rate of severe fibrosis between different types of tumor morphology. Further analysis is, therefore, needed to assess the relationship between fibrosis and tumor morphology due to the small sample size of our study.
Paradoxical movement hinders accurate dissection of the submucosa during colorectal ESD and causes coagulation of the muscularis propria [13, 29]. A longer procedure time encourages paradoxical movement due to an increased accumulation of air in the abdomen [12]. We evaluated paradoxical movement during the procedure when the tip of the colonoscope retracted, even when the endoscopist was able to advance the endoscope appropriately from outside of the patient [25]. Of note, paradoxical movement was not displayed as an image in the present study, such as via magnetic endoscopic imaging (MEI), and instead depended on the sense of endoscopist. However, as endoscopists do occasionally encounter redundant colon during the procedure and must manage paradoxical movements during colorectal ESD, this is not a negligible factor. To compensate for this situation, Ohya et al. [30] reported that a balloon overtube which assists ESD for colorectal lesions could improve access to the lesion and facilitate the scope of manipulation. In these situations, adjunctive devices such as balloon overtubes, including single or double balloon endoscopy, might be useful.
Several limitations to the present study warrant attention. Our study included a relatively small sample size and was retrospective, with a single-center design. Further, as colorectal ESD was performed by one experienced endoscopist in this study, generalizability is limited. A larger patient population examined by more endoscopists in a prospective manner will be required to fully evaluate the factors of incomplete or difficult colorectal ESD.
In conclusion, severe fibrosis was a significant risk factor for both incomplete resection and difficult colorectal ESD. Relatively large tumor size and paradoxical movement were significant risk factors for difficult colorectal ESD, and lengthy procedure time might contribute to incomplete resection. These factors might facilitate development of strategies for treating colorectal ESD, and the use of ancillary devices might enable dissection of severe fibrosis. The development of such devices or methods to overcome these factors is urgently required.
References
Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, Minatsuki C, Yamamichi N, Koike K (2010) Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 42:723–729
Toyonaga T, Man-i M, East JE, Nishino E, Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T, Hirooka T, Fujita T, Inokuchi H, Azuma T (2013) 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc 27:1000–1008
Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W (2014) Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 17(1):130–136
Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S (2009) Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 41:679–683
Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T (2011) Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 54:1307–1312
Kim ES, Cho KB, Park KS, Lee KI, Jang BK, Chung WJ, Hwang JS (2011) Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy 43:573–578
Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisasbe T, Matsuda T, Ishikawa H, Sugihara KI (2013) Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 27(9):3262–3270
Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D (2010) A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 72:1217–1225
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2007) Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association 5:678–683 quiz 645
Lee EJ, Lee JB, Choi YS, Lee SH, Lee DH, Kim do S, Youk EG (2012) Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc 26:1587–1594
Matsumoto A, Tanaka S, Oba S, Kanao H, Oka S, Yoshihara M, Chayama K (2010) Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol 45:1329–1337
Inada Y, Yoshida N, Kugai M, Kamada K, Katada K, Uchiyama K, Handa O, Takagi T, Konishi H, Yagi N, Naito Y, Wakabayashi N, Yanagisawa A, Itoh Y (2013) Prediction and treatment of difficult cases in colorectal endoscopic submucosal dissection. Gastroenterol Res Pract 2013:523084
Yoshida N, Wakabayashi N, Kanemasa K, Sumida Y, Hasegawa D, Inoue K, Morimoto Y, Kashiwa A, Konishi H, Yagi N, Naito Y, Yanagisawa A, Yoshikawa T (2009) Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy 41:758–761
Yoon JY, Kim JH, Lee JY, Hong SN, Lee SY, Sung IK, Park HS, Shim CS, Han HS (2013) Clinical outcomes for patients with perforations during endoscopic submucosal dissection of laterally spreading tumors of the colorectum. Surg Endosc 27:487–493
Tanaka S, Oka S, Chayama K (2008) Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol 43:641–651
Inoue H (2003) The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc 58:S3–43
Saito Y, Kawano H, Takeuchi Y, Ohata K, Oka S, Hotta K, Okamoto K, Homma K, Uraoka T, Hisabe T, Chang DK, Zhou PH (2012) Current status of colorectal endoscopic submucosal dissection in Japan and other Asian countries: progressing towards technical standardization. Dig Endosc: official journal of the Japan Gastroenterological Endoscopy Society 24(Suppl 1):67–72
Lee EJ, Lee JB, Lee SH, Kim do S, Lee DH, Lee DS, Youk EG (2013) Endoscopic submucosal dissection for colorectal tumors—1,000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc 27:31–39
(1983) General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Part II. Histopathological classification. Japanese Research Society for Cancer of the Colon and Rectum. Jpn Surg 13:574-598
Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, Iwashita A, Ajioka Y, Watanabe H, Watanabe T, Muto T, Nagasako K (2004) Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 39:534–543
Nishiyama H, Isomoto H, Yamaguchi N, Ishii H, Fukuda E, Machida H, Nakamura T, Ohnita K, Shikuwa S, Kohno S, Nakao K (2010) Endoscopic submucosal dissection for laterally spreading tumours of the colorectum in 200 consecutive cases. Surg Endosc 24:2881–2887
Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K (2007) Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 66:100–107
Oka S, Tanaka S, Takata S, Kanao H, Chayama K (2012) Usefulness and safety of SB knife Jr in endoscopic submucosal dissection for colorectal tumors. Dig Endosc: official journal of the Japan Gastroenterological Endoscopy Society 24(Suppl 1):90–95
Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D (2007) Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc 66:966–973
Hawari R, Pasricha PJ (2007) Going for the loop: a unique overtube for the difficult colonoscopy. J Clin Gastroenterol 41:138–140
Sato K, Yokouchi Y, Saida Y, Ito S, Kitagawa T, Maetani I (2012) A small cell neuroendocrine carcinoma of the rectum diagnosed by colorectal endoscopic submucosal dissection. J Gastrointest Liver Dis JGLD 21:128
Tanaka S, Terasaki M, Kanao H, Oka S, Chayama K (2012) Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig Endosc: official journal of the Japan Gastroenterological Endoscopy Society 24(Suppl 1):73–79
Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K (2007) Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy 39:418–422
Yoshida N, Yagi N, Naito Y, Yoshikawa T (2010) Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol WJG 16:1688–1695
Ohya T, Ohata K, Sumiyama K, Tsuji Y, Koba I, Matsuhashi N, Tajiri H (2009) Balloon overtube-guided colorectal endoscopic submucosal dissection. World J Gastroenterol WJG 15:6086–6090
Acknowledgments
The authors wish to thank the endoscopy staff for their assistance.
Disclosures
Koichiro Sato, Sayo Ito, Tomoyuki Kitagawa, Mitsuru Kato, Kenji Tominaga, Takeshi Suzuki, and Iruru Maetani have no conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, K., Ito, S., Kitagawa, T. et al. Factors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumors. Surg Endosc 28, 2959–2965 (2014). https://doi.org/10.1007/s00464-014-3558-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3558-y