Abstract
Purpose
Self-expandable metallic stents (SEMS) may be used in acute, obstructing, left-sided colorectal cancer (CRC) to avoid high-risk emergency surgery. However, the data regarding the long-term effects of SEMS as a bridge to surgery are limited and contradictory. Our aim is to analyze the long-term oncological outcomes of SEMS compared with surgery.
Methods
Between January 2006 and November 2013, a total of 855 patients with stage III CRC were regularly followed at the CRC clinic of Severance Hospital, Seoul, Korea. We retrospectively evaluated their 5-year disease-free survival (DFS), 5-year overall survival (OS), and 5-year cancer-specific survival (CSS).
Results
There were 94 patients in the SEMS group, 17 in the emergent-surgery group, and 744 in the elective-surgery group. In the short term, the rate of permanent stoma formation was significantly higher in the emergent-surgery group than in the SEMS group (p = 0.030), although the median hospital stay and overall complication rate were comparable. During the long-term follow-up period, oncological outcomes including 5-year DFS (70.2 vs 52.9%; p = 0.210), OS (70.2 vs 52.9%; p = 0.148), and CSS (79.8 vs 70.6%; p = 0.342) were not different between the SEMS group and the emergent-surgery group. Multivariate analysis showed emergent operation to be a significant risk factor of DFS (hazard ratio [HR], 3.117; 95% confidence interval [CI], 1.498–6.489; p = 0.002).
Conclusions
Preoperative SEMS insertion does not adversely affect long-term oncological outcomes or patient survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is among the most common malignancies worldwide; in Korea, the incidence of CRC has increased rapidly in recent decades [1, 2]. At the time of diagnosis, about 10 to 30% of patients with CRC present with obstructive symptoms that require emergent surgical decompression [3]. However, emergent surgery for CRC is associated with higher rates of complications and higher mortality than elective surgery, indicating that emergent surgery should be avoided if possible [4, 5]. In recent years, self-expandable metallic stents (SEMS) have been used for both palliative and preoperative management of malignant colorectal obstruction [6,7,8]. As a bridge to surgery, the use of stents is associated with lower short-term overall morbidity and lower rates of temporary and permanent stomas than emergent surgery. The stents restore luminal patency and allow for elective surgery with primary anastomosis in most patients [9,10,11].
Although stents have apparent short-term benefits, there have been concerns about the long-term oncological outcomes of preoperative stenting. Many studies report higher rates of disease recurrence in patients who receive SEMS placement than in those who undergo emergent surgery [12,13,14]. In theory, SEMS insertion is an endoscopic procedure that could cause tumor-cell dissemination from either the insertion itself or tumor perforation [15]. Therefore, SEMS placement as a bridge to surgery is not recommended as a first-line treatment unless a patient has an increased risk of postoperative mortality according to the new European Society of Gastrointestinal Endoscopy (ESGE) guidelines [16].
Other studies, however, show the opposite result; preoperative stenting does not adversely affect oncological outcomes [17, 18]. Therefore, scientific debate continues about the best possible strategy for managing malignant colorectal obstruction in patients with a potentially curable CRC. Even the most recent meta-analysis could not draw a concrete conclusion because of a paucity of studies and their heterogeneous methods [19]. Our aim is to evaluate the long-term oncological outcomes of stenting as a bridge to surgery in patients with obstructing left-sided CRC.
Patients and methods
Patients
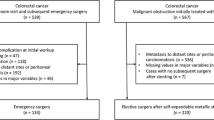
We retrospectively analyzed patients who underwent curative resection of left-sided CRC and who were diagnosed with stage III disease between January 2006 and November 2013 at Severance Hospital, Seoul, Korea.
Colonic obstruction was defined as the presence of symptoms or signs of obstruction, such as abdominal distension, pain, tenderness, or vomiting, with radiological evidence, including the results of plain abdominal x-rays, abdominopelvic computed tomography (CT), or the inability to pass an endoscope beyond the malignant lesion. Left-sided CRC was defined as the presence of cancer from the descending colon to the rectum. The exclusion criteria were incomplete medical records, a history of familial polyposis syndrome or Lynch syndrome, known inflammatory bowel disease, or a follow-up period < 6 months.
Patients were classified into three groups based on the first procedure performed either at or after the time of CRC diagnosis: a SEMS group, an emergent-surgery group with colonic obstruction, and an elective-surgery group without colonic obstruction.
We retrospectively examined and collected the following data from the electronic medical records: patient demographics; tumor characteristics; treatment parameters including type of surgery, stoma formation rate, and morbidity; administration of adjuvant therapy; disease recurrence; and survival. Each patient’s physiologic status was assessed using the American Society of Anesthesiologists (ASA) score. Tumor stages were defined according to the TNM Classification of Malignant Tumors published by the National Comprehensive Cancer Network.
Informed consent was obtained from all patients before the procedure. This study was approved by the institutional review board of Severance Hospital, Yonsei University (Seoul, Korea) and was conducted in accordance with the ethical principles of the Declaration of Helsinki.
SEMS insertion and surgery
The insertion of SEMS was performed by experienced gastroenterologists [20]. After the obstructing tumor was identified by endoscopy, a catheter with a guidewire was introduced through the stricture under combined endoscopic and fluoroscopic guidance. Once the catheter was passed through the lesion, contrast medium was then injected to determine the length and morphology of the malignant stricture. With the guidewire in place, the SEMS was advanced through the working channel of the endoscope until the stent was positioned across the stricture. Upon releasing the delivery catheter, stent deployment started proximally and progressed distally with continued endoscopic and fluoroscopic monitoring. The Niti-S stent (Taewoong Medical), HANARO stent (M.I. Tech), or WallFlex Colonic Stents (Boston Scientific) were used in all cases. Plain abdominal x-rays were obtained after SEMS insertion and again the following day.
We defined technical success as successful stent deployment across the entire length of the stricture, acquisition of stent patency, and radiologic relief of obstruction, and clinical success as the relief of obstructive symptoms.
In the elective- and emergent-surgery groups, the choice of primary anastomosis, stoma formation, and surgical approach (laparoscopic vs open) was determined at the surgeon’s discretion based on their experience, the patient’s clinical condition, and the intraoperative findings. Bowel resection was performed using low anterior resection, anterior resection, left hemicolectomy, or Hartmann’s operation.
Outcome measures
The primary outcomes of this study were 5-year disease-free survival (DFS) and disease recurrence (local or distant). The secondary outcomes were 5-year overall survival (OS) and cancer-specific survival (CSS). Disease recurrence was established using radiological imaging or a histological tissue diagnosis, if possible. The DFS was defined as the time from diagnosis until documented recurrence or death from any cause, CSS was defined as the time to cancer-specific death, and OS was defined as the time to death from any cause or the time to the last follow-up visit.
Statistical analysis
Means and standard deviations, or medians and ranges, were calculated for all continuous variables, as appropriate. Categorical variables were expressed as percentages, and statistical analyses were performed to compare the groups of variables. Either one-way ANOVA testing or the Kruskal-Wallis test was used to compare continuous variables, and either the chi-square test or Fisher’s exact test was used for categorical variables, as appropriate. Kaplan-Meier methods were used to estimate the 5-year DFS, OS, and CSS in the SEMS, emergent-surgery, and elective-surgery groups. Survival curves were compared using the log-rank test. To identify the risk factors for 5-year DFS, OS, and CSS, univariate and multivariate analyses using Cox proportional hazards models were performed, adjusting for various confounders. A p value of < 0.05 was considered statistically significant. All statistical analyses were performed using Statistical Package for the Social Sciences, version 23 (SPSS Inc., Armonk, NY, USA).
Results
Baseline characteristics
A total of 855 patients were treated for left-sided, stage III CRC at our institution between January 2006 and November 2013. A total of 744 patients had no colonic obstruction (the elective-surgery group). Of the 111 patients with left-sided obstruction, 94 underwent SEMS as a bridge to surgery followed by elective surgical resection, and 17 underwent emergent surgery. The patient demographics and tumor characteristics are summarized in Table 1. Patient age, the primary tumor site, and pathological findings including staging, harvested lymph node count, lymphovascular invasion, and tumor size were different between the three groups. However, when comparing only the SEMS group and the emergent-surgery group, no differences in these baseline variables was observed except for the lymphovascular invasion and tumor size: lymphovascular invasion was significantly more frequent (p = 0.016) and tumor size was larger in the SEMS group (p = 0.007).
Procedure-related outcomes
Stent placement was technically successful in 92 of 94 patients in the SEMS group, and the technical failures in two patients were due to unsuccessful cannulation over the guidewire. Stent insertion was clinically successful in 90 of 92 patients. One patient suffered immediate perforation after stent insertion and one patient had insufficient decompression with the stent. Among the 90 patient who achieved clinical success, three patients experienced complications of perforation (1 patient) and anal pain (2 patients). Including immediate perforation after stent insertion, stent-related perforations before surgery occurred in two patients (2.1%) in the SEMS group, all of whom underwent emergent surgery. Subsequent surgery was performed at a mean of 9.7 days after SEMS placement, and emergent surgery was performed at a mean of 3.9 days after diagnosis of CRC.
There were no significant differences in perioperative outcomes between the three groups except for the type of resection performed (Table 2). The short-term outcomes of the SEMS group and the emergent-surgery group were comparable (type of surgery, median hospital stay, and overall complications). However, the rate of permanent stoma formation was significantly higher in the emergent-surgery group than in the SEMS group (23.5 vs 5.3%; p = 0.030).
Long-term oncological outcomes
The median follow-up period was 58.2 months (range, 5–130 months), 50.4 months (range, 11–117 months), and 87.9 months (range, 4–139 months) in the SEMS group, emergent-surgery group, and elective-surgery group, respectively. During the follow-up period, the 5-year DFS remained higher among patients in the SEMS group than in the emergent-surgery group, but was lower than the DFS seen in the elective-surgery group (70.2, 52.9, and 77.3%, respectively; p = 0.025). No statistically significant difference was observed in 5-year DFS between the SEMS group and the emergent-surgery group (p = 0.210; Fig. 1a). Thirty patients (31.9%) experienced disease recurrence after colorectal stenting (6 with local recurrence, 24 with distant metastasis), compared with 8 patients (47.1%) after emergent surgery (1 with local recurrence, 7 with distant metastasis) and 182 patients (24.5%) after elective surgery (27 with local recurrence, 155 with distant metastasis). Disease recurrence rates were comparable (Table 3). No statistically significant difference was observed for either the 5-year OS (70.2 vs 52.9%; p = 0.148, Fig. 1b) or the 5-year CSS (79.8 vs 70.6%; p = 0.342; Fig. 1c) between the SEMS group and the emergent-surgery group.
Kaplan-Meier analysis (log-rank tests) comparing (a) disease-free survival at 5 years, SEMS vs. emergent surgery, p = 0.210; elective surgery vs. emergent surgery, p = 0.017; elective surgery vs. SEMS, p = 0.090. b Overall survival at 5 years, SEMS vs. emergent surgery, p = 0.148; elective surgery vs. emergent surgery, p < 0.001; elective surgery vs. SEMS, p = 0.002, and c cancer-specific survival at 5 years, SEMS vs. emergent surgery, p = 0.342; elective surgery vs. emergent surgery, p = 0.005; elective surgery vs. SEMS, p = 0.001
In addition, multivariate analysis correcting for known risk factors for 5-year DFS including patient age, sex, pathological tumor stage, node stage, tumor size, primary tumor differentiation, and the presence of lymphovascular invasion showed emergent surgery to be a significant risk factor of DFS (hazard ratio, 3.117; 95% confidence interval, 1.498–6.489; p = 0.002; Table 4). Similarly, in multivariate analysis, emergency surgery was identified as a risk factor for OS (HR, 2.825; 95% CI, 1.362–5.859; p = 0.005; supplementary data 1) and CSS (HR, 3.662; 95% CI, 1.437–9.336; p = 0.007; supplementary data 2).
Discussion
We found that SEMS placement as a bridge to surgery does not adversely affect long-term oncological outcomes in patients with curable CRC. There is no difference in oncological outcomes such as disease recurrence and survival between patients who undergo SEMS vs emergent surgery. Remarkably, after adjusting for confounding factors, multivariate analysis reveals that emergency surgery, not SEMS insertion, was a significant risk factor for disease recurrence and survival in patients with obstructive, left-sided, stage III CRC.
Bowel obstruction in patients with CRC may lead to perforation, which often becomes life- threatening and leads to a poor prognosis [21]. As the number of elderly patients with multiple comorbidities is increasing, the management of left-sided obstruction in CRC is becoming complicated and difficult [2, 22]. Since the first report of the preoperative use of colorectal stenting by Tejero in 1994 [23], many studies have proven the short-term benefits of SEMS as a bridge to surgery, with lower rates of complications and stoma formation and higher rates of primary anastomosis than emergent surgery [23]. However, the data are contradictory for any long-term oncological benefits of colorectal stenting in curable patients.
One of the major concerns of SEMS placement is a potential negative effect on oncological outcomes with the risk of disseminating a localized tumor through bowel perforation. Bowel perforation may be caused by excessive manipulation of the guidewire, injury of friable tumor tissue resulting from the radial force of the stent, and erosion of the colonic wall by the end of the stent [24]. Indeed, many articles report higher rates of disease recurrence in patients undergoing SEMS insertion [12,13,14], and Sloothaak et al. [14] found worse DFS in a subgroup of patients with stent-related perforation. Thus, the current ESGE guidelines suggest that colorectal stenting with curative intent might be considered as an alternative treatment in patients whose perioperative risks for emergent surgery outweigh the oncological risks of stent insertion (e.g., patients older than 70 years or with an ASA score > III) rather than in all patients. A recent nationwide Danish cohort study found that the use of SEMS as a bridge to surgery is associated with an increased risk of CRC recurrence after 5 years, supporting the recommendations of the ESGE guidelines [16, 25].
Similar to those of previous studies, our results demonstrate the short-term advantages of SEMS placement, i.e., a lower rate of permanent stoma formation, though the overall complication rate is not different from emergent surgery [9, 11]. On the other hand, the DFS and OS seen in patients with SEMS insertion is not inferior to emergent surgery, and emergency surgery was significantly associated with disease recurrence and survival in the multivariate analysis. The discrepancy of this result from those of prior studies can be explained by several factors. Sabbagh et al. [13] reported that OS is lower in SEMS, but a subsequent study suggested that differences in the pathologic baselines between groups could affect the oncological outcomes [26]. Indeed, the presence of ulcerations at or near the tumor, perineural invasion, and lymph node invasion are more frequently seen in the SEMS groups of Sabbagh’s study. Our data also show a higher rate of lymphovascular invasion and a larger tumor size in the SEMS group, which might be an obstacle to interpreting the oncological outcomes of SEMS insertion. In addition, several studies have found that the tendency toward no difference in OS between groups becomes more distinct in patients with stage II and stage III CRC [18, 27]. However, previous studies did not limit patient selection by CRC stage. In stage II disease, the obstruction itself might be a risk factor for recurrence and may affect survival; we therefore included a homogenous population of only stage III CRC patients and compared the oncological outcomes after SEMS insertion and emergent surgery with those obtained from elective surgery.
There have been recent studies supporting our data that preoperative SEMS insertion has comparable long-term outcomes to emergent surgery [10, 18, 27, 28]. It is noteworthy that SEMS-associated perforations occurred in 2.1% of patients in our study, a lower rate than previously reported (6.9–23.1%) [14, 29, 30]. In addition, our technical and clinical success rates of 97.9 and 97.8% are higher than those seen in a previous meta-analysis (70.0 and 69.0%) [29], implying that proficient and adequate SEMS placement could reduce the risk of local recurrence by avoiding the risk of perforation. In the sensitivity analysis of a meta-analysis by Matsuda et al. [19], OS is better in the SEMS group than in the emergent-surgery group when a large number of patients are involved or the success rate of SEMS insertion is high. In this context, the quality of individual centers could be an important factor, and colorectal stenting as a bridge to surgery might be helpful if SEMS insertion is performed by experienced gastroenterologists at a high-volume center.
Our study has the innate limitations of a retrospective, cross-sectional, case-control study performed at a single tertiary university hospital. In addition, there may be selection bias inherent in deciding whether to insert stents as a bridge to surgery or to perform emergent surgery in patients with obstructing, left-sided, stage III CRC. However, our study has strong points as well; all interventions were performed by a highly specialized surgical team and by gastroenterologists, and we had access to detailed long-term follow-up data. Also, unlike previous meta-analyses [31, 32], our SEMS group had comparable short-term outcomes compared with patients undergoing elective or emergent surgery. Our small sample size and the competence of our surgical team could be the cause of this observation. Finally, the number of patients included in the emergent-surgery group is too small to draw a concrete conclusion. Further large-scale, prospective study is necessary to prove the long-term oncological outcomes of SEMS as part of a curative regimen.
In conclusion, preoperative SEMS insertion has comparable long-term oncological outcomes to emergent surgery. Our findings suggest that, when performed by experienced gastroenterologists, placement of SEMS as a bridge to surgery could be considered as an alternative option for the management of malignant colorectal obstruction.
References
Yoon M, Kim N, Nam B, Joo J, Ki M (2015) Changing trends in colorectal cancer in the Republic of Korea: contrast with Japan. Epidemiol Health 37:e2015038. https://doi.org/10.4178/epih/e2015038
Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, Kim DY, Oh JH (2012) Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat 44(4):219–226. https://doi.org/10.4143/crt.2012.44.4.219
Fan YB, Cheng YS, Chen NW, Xu HM, Yang Z, Wang Y, Huang YY, Zheng Q (2006) Clinical application of self-expanding metallic stent in the management of acute left-sided colorectal malignant obstruction. World J Gastroenterol 12(5):755–759
Sjo OH, Larsen S, Lunde OC, Nesbakken A (2009) Short term outcome after emergency and elective surgery for colon cancer. Color Dis 11(7):733–739. https://doi.org/10.1111/j.1463-1318.2008.01613.x
McArdle CS, Hole DJ (2004) Emergency presentation of colorectal cancer is associated with poor 5-year survival. Br J Surg 91(5):605–609. https://doi.org/10.1002/bjs.4456
Saida Y, Sumiyama Y, Nagao J, Uramatsu M (2003) Long-term prognosis of preoperative “bridge to surgery” expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum 46(10 Suppl):S44–S49. https://doi.org/10.1097/01.DCR.0000087483.63718.A2
Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballon P, Moreno-Azcoita M (2002) Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum 45(3):401–406
Ng KC, Law WL, Lee YM, Choi HK, Seto CL, Ho JW (2006) Self-expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left-sided colorectal cancer: a case-matched study. J Gastrointest Surg 10(6):798–803. https://doi.org/10.1016/j.gassur.2006.02.006
Jimenez-Perez J, Casellas J, Garcia-Cano J, Vandervoort J, Garcia-Escribano OR, Barcenilla J, Delgado AA, Goldberg P, Gonzalez-Huix F, Vazquez-Astray E, Meisner S (2011) Colonic stenting as a bridge to surgery in malignant large-bowel obstruction: a report from two large multinational registries. Am J Gastroenterol 106(12):2174–2180. https://doi.org/10.1038/ajg.2011.360
Arezzo A, Passera R, Lo Secco G, Verra M, Bonino MA, Targarona E, Morino M (2017) Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc 86:416–426. https://doi.org/10.1016/j.gie.2017.03.1542
Huang X, Lv B, Zhang S, Meng L (2014) Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: a meta-analysis. J Gastrointest Surg 18(3):584–591. https://doi.org/10.1007/s11605-013-2344-9
Gorissen KJ, Tuynman JB, Fryer E, Wang L, Uberoi R, Jones OM, Cunningham C, Lindsey I (2013) Local recurrence after stenting for obstructing left-sided colonic cancer. Br J Surg 100(13):1805–1809. https://doi.org/10.1002/bjs.9297
Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac E, Regimbeau JM (2013) Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg 258(1):107–115. https://doi.org/10.1097/SLA.0b013e31827e30ce
Sloothaak DA, van den Berg MW, Dijkgraaf MG, Fockens P, Tanis PJ, van Hooft JE, Bemelman WA, Collaborative Dutch Stent-In Study Group (2014) Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg 101(13):1751–1757. https://doi.org/10.1002/bjs.9645
Han SH, Lee JH (2014) Colonic stent-related complications and their management. Clin Endosc 47(5):415–419. https://doi.org/10.5946/ce.2014.47.5.415
van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jimenez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A, European Society of Gastrointestinal E (2014) Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Gastrointest Endosc 80(5):747–761 e741–775. https://doi.org/10.1016/j.gie.2014.09.018
Arezzo A, Balague C, Targarona E, Borghi F, Giraudo G, Ghezzo L, Arroyo A, Sola-Vera J, De Paolis P, Bossotti M, Bannone E, Forcignano E, Bonino MA, Passera R, Morino M (2016) Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc 31:3297–3305. https://doi.org/10.1007/s00464-016-5362-3
Tung KL, Cheung HY, Ng LW, Chung CC, Li MK (2013) Endo-laparoscopic approach versus conventional open surgery in the treatment of obstructing left-sided colon cancer: long-term follow-up of a randomized trial. Asian J Endosc Surg 6(2):78–81. https://doi.org/10.1111/ases.12030
Matsuda A, Miyashita M, Matsumoto S, Matsutani T, Sakurazawa N, Takahashi G, Kishi T, Uchida E (2015) Comparison of long-term outcomes of colonic stent as “bridge to surgery” and emergency surgery for malignant large-bowel obstruction: a meta-analysis. Ann Surg Oncol 22(2):497–504. https://doi.org/10.1245/s10434-014-3997-7
Baron TH, Rey JF, Spinelli P (2002) Expandable metal stent placement for malignant colorectal obstruction. Endoscopy 34(10):823–830. https://doi.org/10.1055/s-2002-34271
Carraro PG, Segala M, Orlotti C, Tiberio G (1998) Outcome of large-bowel perforation in patients with colorectal cancer. Dis Colon Rectum 41(11):1421–1426
Marley AR, Nan H (2016) Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet 7(3):105–114
Tejero E, Mainar A, Fernandez L, Tieso A, Cuezva JF, San Jose A (1995) New procedure for relief of malignant obstruction of the left colon. Br J Surg 82(1):34–35
Kim SJ, Kim HW, Park SB, Kang DH, Choi CW, Song BJ, Hong JB, Kim DJ, Park BS, Son GM (2015) Colonic perforation either during or after stent insertion as a bridge to surgery for malignant colorectal obstruction increases the risk of peritoneal seeding. Surg Endosc 29(12):3499–3506. https://doi.org/10.1007/s00464-015-4100-6
Erichsen R, Horvath-Puho E, Jacobsen JB, Nilsson T, Baron JA, Sorensen HT (2015) Long-term mortality and recurrence after colorectal cancer surgery with preoperative stenting: a Danish nationwide cohort study. Endoscopy 47(6):517–524. https://doi.org/10.1055/s-0034-1391333
Sabbagh C, Chatelain D, Trouillet N, Mauvais F, Bendjaballah S, Browet F, Regimbeau JM (2013) Does use of a metallic colon stent as a bridge to surgery modify the pathology data in patients with colonic obstruction? A case-matched study. Surg Endosc 27(10):3622–3631. https://doi.org/10.1007/s00464-013-2934-3
Kwak MS, Kim WS, Lee JM, Yang DH, Yoon YS, Yu CS, Kim JC, Byeon JS (2016) Does stenting as a bridge to surgery in left-sided colorectal cancer obstruction really worsen oncological outcomes? Dis Colon Rectum 59(8):725–732. https://doi.org/10.1097/dcr.0000000000000631
Alcantara M, Serra-Aracil X, Falco J, Mora L, Bombardo J, Navarro S (2011) Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 35(8):1904–1910. https://doi.org/10.1007/s00268-011-1139-y
Tan CJ, Dasari BV, Gardiner K (2012) Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg 99(4):469–476. https://doi.org/10.1002/bjs.8689
Sagar J (2011) Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev 11:CD007378. https://doi.org/10.1002/14651858.CD007378.pub2
Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M (2004) Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 99(10):2051–2057. https://doi.org/10.1111/j.1572-0241.2004.40017.x
Khot UP, Lang AW, Murali K, Parker MC (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89(9):1096–1102. https://doi.org/10.1046/j.1365-2168.2002.02148.x
Acknowledgements
The authors thank native English-speaking experts from BioMed Proofreading® LLC for the editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was obtained from all patients before the procedure. This study was approved by the institutional review board of Severance Hospital, Yonsei University (Seoul, Korea) and was conducted in accordance with the ethical principles of the Declaration of Helsinki
Electronic supplementary material
ESM 1
(DOCX 31 kb).
Rights and permissions
About this article
Cite this article
Park, J., Lee, H.J., Park, S.J. et al. Long-term outcomes after stenting as a bridge to surgery in patients with obstructing left-sided colorectal cancer. Int J Colorectal Dis 33, 799–807 (2018). https://doi.org/10.1007/s00384-018-3009-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-018-3009-7