Abstract
Background
Laparoscopic resection of gastric gastrointestinal stromal tumors (GISTs) appears technically feasible and associated with favorable outcomes. Tumor size plays an important role in surgical approach, with laparotomy tending to be used to treat larger tumors. This study evaluated the technical feasibility, safety, and oncologic efficacy of laparoscopic surgery for GISTs ≥5 cm in diameter.
Methods
One hundred forty patients who underwent resection of primary gastric GIST at our institution from January 2007 to December 2012 were identified. Twenty-three patients with tumor larger than 5 cm in diameter treated by laparoscopic resection and were randomly matched (1:1) by tumor size (±1 cm) to patients with open resection. Clinical and pathologic variables and surgical outcomes for each surgical type were identified and compared.
Results
There were no significant differences in clinicopathologic characteristics between the two groups. Laparoscopic group was superior to open group in operation time, blood loss, time to ground activities, time to first flatus, times to liquid diet, and postoperative stay (P < 0.05). Number of transfusions and time to semi-liquid diet, however, did not differ between groups. There was no operative mortality, and the postoperative complications were similar. Fifteen patients in the laparoscopic group and 17 patients in the open group received adjuvant treatment with imatinib. Recurrence or metastasis occurred in eight cases (three in the laparoscopic group and five in the open group). No significant difference in long-term disease-free survival was found between the two groups (P > 0.05).
Conclusion
When performed by experienced surgeons, laparoscopic resection for gastric GISTs larger than 5 cm is a safe and effective minimally invasive surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastrointestinal stromal tumor (GIST) is the most common type of mesenchymal tumor of the gastrointestinal (GI) tract [1], but occurs only rarely, with an incidence of approximately 10–20 neoplasms per 1 million cases annually [2]. GISTs occur most commonly in the stomach (60–70 %) [1], and surgical resection is the only possible curative therapy. Because GISTs grow by expansion rather than diffuse infiltration, and seldom invade nearby lymph nodes, routine lymph node dissection is usually not warranted [3–5] and minimally invasive surgery (MIS) is feasible. Advances in equipment and techniques now allow many surgical procedures to be performed through tiny abdominal incisions. When performed by skilled operators, MIS results in dramatically shorter hospital stays and less postoperative discomfort. Therefore, gastric GISTs resection is particularly amenable to laparoscopic surgery. Previously, the GIST Consensus Conference (2004) recognized laparoscopic resection as an appropriate operative intervention for small tumors [6], and the last decade has brought dramatic changes in laparoscopic surgery in this area. Several case series have demonstrated the safety and feasibility of laparoscopic resection of larger gastric GISTs [7–9]; however, the oncologic benefits of this technology have rarely been reported for tumors larger than 5 cm. In the present study, we retrospectively reviewed detailed data for patients who underwent laparoscopic surgery or traditional open surgery for resection of GIST at our center between 2007 and 2012. Clinical data, benefits of operation, perioperative events, and oncologic outcomes were evaluated for associations with type of surgery in a matched-cohort study.
Materials and methods
Materials
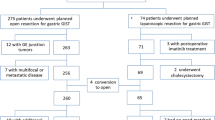
Between January 2007 and December 2012, 140 patients with primary gastric GISTs were treated with radical resection at the Department of Gastric Surgery, Fujian Medical University Union Hospital. A retrospective analysis was performed, using a prospectively maintained comprehensive database, to determine the technical pitfalls of the procedure. Out of the 140 patients, 23 who had a tumor larger than 5 cm in diameter (range, 5.0–9.7 cm) underwent laparoscopic resection and were matched to patients in the open group by tumor size (±1 cm) and whether neoadjuvant imatinib was administered. When more than one patient matched, the patient whose date of surgery was closest was selected. Clinical and pathological data were obtained from patients’ medical records. Clinical diagnosis of GIST was based on endoscopic features such as an intact mucosa, a predominantly submucosal location, and in some cases, a pedunculated appearance. Most of the patients also underwent preoperative abdominal computed tomography (CT) as part of their initial diagnostic workup or to assess for the presence of distant metastases or local tumor invasion. Hospital charts were reviewed, and various outcome measures recorded, including operative time, estimated operative blood loss, morbidity, time to oral intake, and postoperative hospital stay. All GISTs were pathologically confirmed by dedicated sarcoma pathologists at our center. Mitotic rate was defined as number of mitoses per 50 high-power fields (HPFs), and tumor size was defined as the maximal tumor dimension in the resected specimen. Estimated blood loss was obtained from operative or anesthesia records. When not precisely quantified but described as negligible or minimal, it was assigned a value of 5 mL. Patients’ management pathways followed the National Comprehensive Cancer Network (NCCN) guidelines and European Society for Medical Oncology (ESMO) guidelines [10, 11]. If R0 surgery was not feasible, or if it could be achieved through less mutilating surgery with cytoreduction, or the surgeon believed the surgery could be made safer for a patient, neoadjuvant imatinib therapy was recommended. Neoadjuvant therapy with imatinib was administered to one patient in each group. Risk stratification for relapse was performed on the basis of prognostic factors, which included: mitotic rate, tumor size, tumor site, and surgical margins (including whether tumor rupture occurred) [12]. Routine follow-up of patients consisted of physical examination, laboratory tests, chest radiography, abdominopelvic ultrasonography (USG) or computed tomography (CT), and an annual endoscopic examination. Survival periods were calculated from the time of surgery until death related to the disease or not, or were right-censored at final follow-up.
Surgical procedure
Tumors were classified according to tumor location (Fig. 1). Indications for the types of surgery by tumor location were as follows: Tumor in the upper part of the stomach near the esophagus (gastroesophageal junction)—proximal subtotal gastrectomy (STG); Tumor located at fundus of stomach and greater curvature of gastric body—Partial gastrectomy; Tumor in the lower part of the stomach (near the pylorus)—Distal STG; Tumor involved two or more sections—TG.
Open resection was typically performed through a 15–20 cm midline incision. For the laparoscopic approach, the patient was placed in supine position with his or her legs spread apart and general anesthesia was given. After pneumoperitoneum was established at 12 mmHg, a 10-mm trocar for the laparoscope was inserted below the umbilicus. The stomach and peritoneal cavity were inspected using a 30° forward oblique laparoscope to rule out invasion of adjacent organs and peritoneal seeding. A 12-mm port was inserted percutaneously in the left upper quadrant as the dominant hand port. A 5-mm trocar was placed in the contralateral side. Another two 5-mm trocars were inserted, one each in the left and right lower quadrants. The surgeon stood to the left side of the patient, the first assistant stood to the right, and another assistant who helped to manipulate the laparoscope stood between the patient’s legs. Occasionally, gastroscopy was used to assist with identification and extent of the tumor. Partial gastrectomy was usually achieved using an Endo GIATM stapler, and the tumor specimen was extracted using a bag. Laparoscopic proximal or distal STG and TG was performed via a 6–10-cm epigastric incision.
Statistics
Statistical analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL) for Student’s t test or chi-square test, and cumulative survival was using the Kaplan-Meier method and log rank test. Chi-square analysis was performed to find associations between poor outcomes and factors such as age, tumor size, mitotic index, necrosis, ulcerations, nodal disease, and CD117 and CD34 expression. Cox proportional hazard model was used for multivariate analysis. A P value <0.05 was considered statistically significant.
Results
Patients characteristics
Characteristics of the 46 case-matched patients (23 laparoscopic vs. 23 open) were listed in Table 1. There were 27 males and 19 females whose age ranged from 38 to 75 years (62.7±10.1 years). The mean tumor sizes in the laparoscopic group and open group were 7.2 cm (range, 5.0–9.7 cm) and 7.3 cm (range, 5.0–10.5 cm), respectively. According to proposed classification of GISTs by relative risk of malignancy [13], 29 cases (67.4 %) were classified as intermediate risk and 17 cases (32.6 %) as high risk. The numbers of cases of upper, middle, and lower third gastric GISTs were 15, 5, and 3, respectively, in laparoscopic group, whereas in open group the numbers were 9, 11, and 3. There were no statistically significant differences in the major demographic parameters between the two groups (Table 1).
Operative outcomes
No tumor rupture occurred in the laparoscopic group. The number of patients transfused (P = 0. 489) and time to semi-liquid diet (P = 0.083) between the two groups was similar. However, the laparoscopic group was superior to open surgery group in operation time (P < 0.001), blood loss (P = 0.006), time to ground activities (P = 0.004), time to first flatus (P = 0.018), times to liquid diet (P = 0.011), and postoperative hospital stay (P = 0.001). There was one conversion to open surgery (4.3 %), because the operation was performed by surgeons during the initial part of their learning curve for laparoscopy. The incidence of postoperative complications did not differ between the two groups (two patients in the laparoscopy group [8.7 %] vs. three patients in the open group [13.0 %]; P = 0.636). No patient died during their hospital stay in either group (Table 2).
Adjuvant treatment and oncologic outcomes
Fifteen patients in the laparoscopic group and 17 patients in the open group underwent adjuvant imatinib therapy (8–24 months). One patient in each group received neoadjuvant imatinib; for 3 months preoperatively in the laparoscopic group and for 6 months in the open group (Table 3).
Median follow up duration for the entire cohort was 34.0 months (range, 6–78 months). Eight patients had recurrence, three with local recurrence and five with metastasis. Three patients, all defined as high risk, died of the disease: one in the laparoscopic group and two in the open group (Table 3). There was no significant difference in disease-free survival between the two groups (Fig. 2).
Assessment of recurrent risk
Recurrence and metastasis during the follow-up period were considered poor outcomes, and prognostic factors such as age, tumor size, laparoscopic vs. open surgery, mitotic index, antigen expression, necrosis, ulceration, and macroscopic surgical margin were examined. Univariate analysis demonstrated a significant correlation between poor outcomes and tumor size, mitotic index, tumor necrosis, and adjuvant treatment. However, further multivariate analysis revealed that only the tumor mitotic index was an independent predictive variable affecting survival (hazard ratio = 12.325; confidence interval, 2.131–36.523, P < 0.001).
Discussion
The stomach is by far the most common site of GISTs (52–60 % of cases), with the proximal stomach involved in about two-thirds of those patients [13–15], and gastric GISTs are becoming more frequently encountered because of the rising use of upper gastrointestinal endoscopy. Prior to laparoscopic resection of a GIST, it is important that tumor size, tumor location, local infiltration, and metastasis be assessed with endoscopy or endoscopic ultrasound and other imaging methods. Tumor size is widely accepted as a prognostic factor, and can be accurately evaluated before surgery. Mochizuki et al. [16] suggested that only gastric GISTs <5 cm in diameter are suitable for laparoscopic surgery because very large lesions are difficult to resect using endoscopic linear staplers, and that the removal of a GIST requires special care to prevent tumor spillage. However, in a study by Daigle et al. [17], 19 of 23 GIST patients underwent laparoscopic resection of tumors a mean of 3.2 cm in size, with the largest tumor measuring 6.8 cm. There were no episodes of tumor rupture or spillage and no major intraoperative complications. They concluded that Laparoscopic resection is the preferred technique for these patients. Ma et al. [18] also reported that they had successfully performed laparoscopic gastric resection of a tumor 11.5 cm in diameter.
NCCN guidelines were modified in 2007 to reflect the increasing literature on this approach. Under the new guidelines, tumors up to 5 cm in size can be safely approached laparoscopically, and even larger tumors can be considered for a laparoscopic hand-assisted approach [10]. However, only rare direct comparison of laparoscopic and open approaches for gastric GISTs larger than 5 cm had been done. In our study, all tumors were greater than 5 cm in diameter, the largest tumor was 9.7 cm, and were successfully resected laparoscopically without tumor rupture. Only one patient, whose tumor measured 6 cm, underwent conversion to open surgery because it was performed during the initial part of the surgeons’ learning curve for laparoscopy. To maintain the integrity of the tumor under laparoscopy, it is important to avoid grasping the tumor directly and to prevent forceful extraction of the tumor out of the abdominal cavity through the small incision. We believe that the decision to proceed with a laparoscopic approach should be based on a variety of factors, including patient characteristics, tumor size, invasion, and location, and operators’ skills and experience. But a large gastric GIST is not an absolute contraindication for laparoscopic surgery when performed by experienced surgeons.
Because wide surgical margins and lymphadenectomy are usually unnecessary, a minimally invasive technique such as laparoscopic surgery for GISTs has found favor with many surgeons. Pitsinis et al. [19] compared open and laparoscopic resection of GISTs and reported that the median operating time was 132 minutes in the open group and 110 minutes in the laparoscopic group, and that patients who undergone laparoscopic resection had a shorter hospital stay (4 vs. 11 days). Chen et al. [20] compared the outcomes of 16 patients who underwent laparoscopic surgery to those of 40 patients who underwent open surgery, and found that laparoscopic resection had advantages of fewer days to resume diet, shorter postoperative stay, and less analgesia use during the perioperative period. Our size-matched comparison revealed similar short-term results. In addition, there was no statistically difference in the mobility and mortality between the two groups. From this viewpoint, laparoscopy for gastric GISTs larger than 5 cm is a safe and feasible choice.
Information regarding long-term oncologic results of laparoscopic resection of gastric GISTs, which is important to confirm the appropriateness of the use of laparoscopic technique, is scarce. Novitsky et al. [21] reported a case series in which 50 patients underwent laparoscopic or laparoscopic resection of gastric GISTs with mean tumor size of 4.4 cm. All 50 patients had negative resection margins, and 92 % of the patients were disease free during a 36-month follow-up. Karakousis et al. [22] presented a size-matched comparison of laparoscopic resection vs. open resection for GISTs. Oncologic outcomes were similar with no microscopically positive margins and one recurrence in each group during a median follow-up of 34 months. In the present series, we found that there was no significant difference in the survival curve between the two groups. Laparoscopic surgery for gastric GISTs larger than 5 cm achieved survival similar to that of open surgery, although the present study was not a randomized controlled trial and the follow-up period was relatively short.
The prognostic characteristics of surgically treated GISTs have not been clearly defined in the past. However, recent studies have provided a more comprehensive clinical and histologic analysis of GISTs. Factors associated with poor prognosis are age, tumor size, mitotic index, tumor ulceration, and necrosis [23–25] (Table 4). On the other hand, the development of imatinib (Gleevec [Novartis], a tyrosine kinase inhibitor) has been strongly justified in the treatment of metastatic GISTs [26, 27]. More trials and studies have been initiated to assess the role of neoadjuvant and adjuvant therapy in treating non-metastatic disease. The findings of which may help improve the long-term survival of patients undergoing laparoscopic or open resection of GISTs. While neoadjuvant imatinib therapy for gastric GIST is still controversial [28, 29], and there are rare studies on the laparoscopic resection for gastric GISTs after neoadjuvant therapy. If it results in less mutilating surgery and lower risk of tumor bleeding and rupture, preoperative imatinib seems an option for patients with limited disease. There was one patient who received neoadjuvant Gleevec therapy had successfully underwent laparoscopic gastrectomy, the excellent result may provide a new choice for gastric GISTs after neoadjuvant therapy. But a well designed, large sample, multicenter, randomized study is necessary before a definitive clinical benefit can be determined. In our study, univariate analysis demonstrated that tumor size, mitotic index, tumor necrosis, and adjuvant treatment were associated with poorer outcomes. However, further multivariate analysis revealed tumor mitotic index to be the only independent predictor of survival. Surgical method (laparoscopy or open resection) was not found to be associated with poor outcomes. Given the fact that the safety of laparoscopic techniques in colonic and gastric oncologic surgeries has been confirmed by recent trials [30–32], the role of laparoscopic resection for GISTs of the stomach may be further clarified in the near future.
In conclusion, our size-matched comparison has demonstrated that laparoscopic resection of gastric GISTs up to 10 cm results in operative durations, morbidity, and outcomes that are similar to those of open resection but is associated with shorter hospital stays, and that tumor location is clearly an important factor in the selection of an operative approach. Laparoscopic gastric resection selected based on tumor location is a safe and feasible minimally invasive approach for appropriate cases of gastric GISTs and provides a favorable oncologic outcome.
References
Miettinen M, Lasota J (2001) Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 438(1):1–12
Corless CL, Fletcher JA, Heinrich MC (2004) Biology of gastrointestinal stromal tumors. J Clin Oncol 22(18):3813–3825
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231(1):51–58
Woodall CE, Brock GN, Fan J, Byam JA, Scoggins CR, McMasters KM, Martin RC (2009) An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg 144(7):670–678
Kong SH, Yang HK (2013) Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer 13(1):3–18
Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, Le Cesne A, McClure J, Maurel J, Nupponen N, Ray-Coquard I, Reichardt P, Sciot R, Stroobants S, van Glabbeke M, van Oosterom A, Demetri GD (2005) Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol 16(4):566–578
Joensuu H (2008) Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39(10):1411–1419
Basu S, Balaji S, Bennett DH, Davies N (2007) Gastrointestinal stromal tumors (GIST) and laparoscopic resection. Surg Endosc 21(10):1685–1689
Lee JS, Kim JJ, Park SM (2011) Totally laparoscopic resection for a large gastrointestinal stromal tumor of stomach. J Gastric Cancer 11(4):239–242
Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS, Fisher GA, Fletcher CD, Gronchi A, Hohenberger P, Hughes M, Joensuu H, Judson I, Le Cesne A, Maki RG, Morse M, Pappo AS, Pisters PW, Raut CP, Reichardt P, Tyler DS, Van den Abbeele AD, von Mehren M, Wayne JD, Zalcberg J (2007) NCCN task force report: management of patients with gastrointestinal stromal tumor (GIST)-update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5(2):1–29
Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY (2009) ESMO Guidelines Working Group. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 4:64–67
Joensuu H (2008) Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39(10):1411–1419
Matthews BD, Joels CS, Kercher KW, Heniford BT (2004) Gastrointestinal stromal tumors of the stomach. Minerva Chir 59(3):219–231
Selcukbiricik F, Yalçın S, Tural D, Erdamar S, Demir G, Doğusoy G, Mandel NM (2013) Gastrointestinal stromal tumors in Turkey: experiences from three centers. Onkologie 36(1–2):18–24
Blanke CD, Corless CL (2005) State-of-the art therapy for gastrointestinal stromal tumors. Cancer Invest 23(3):274–280
Mochizuki Y, Kodera Y, Fujiwara M, Ito S, Yamamura Y, Sawaki A, Yamao K, Kato T (2006) Laparoscopic wedge resection for gastrointestinal stromal tumors of the stomach: initial experience. Surg Today 36(4):341–347
Daigle C, Meneghetti AT, Lam J, Panton ON (2012) Laparoscopic management of gastrointestinal stromal tumours: review at a Canadian centre. Can J Surg 55(2):105–109
Ma JJ, Hu WG, Zang L, Yan XW, Lu AG, Wang ML, Li JW, Feng B, Zhong J, Zheng MH (2011) Laparoscopic gastric resection approaches for gastrointestinal stromal tumors of stomach. Surg Laparosc Endosc Percutan Tech 21(2):101–105
Pitsinis V, Khan AZ, Cranshaw I, Allum WH (2007) Singlecenter experience of laparoscopic versus open resection for gastrointestinal stromal tumors of the stomach. Hepatogastroenterology 54(74):606–608
Chen YH, Liu KH, Yeh CN, Hsu JT, Liu YY, Tsai CY, Chiu CT, Jan YY, Yeh TS (2012) Laparoscopic resection of gastrointestinal stromal tumors: safe, efficient, and comparable oncologic outcomes. J Laparoendosc Adv Surg Tech A 22(8):758–763
Novitsky YW, Kercher KW, Sing RF, Heniford BT (2006) Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 243(6):738–745
Karakousis GC, Singer S, Zheng J, Gonen M, Coit D, De-Matteo RP, Strong VE (2011) Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol 18(6):1599–1605
Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P (2012) Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 13(3):265–274
Nowain A, Bhakta H, Pais S, Kanel G, Verma S (2005) Gastrointestinal stromal tumors: clinical profile, pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol 20(6):818–824
Fujimoto Y, Nakanishi Y, Yoshimura K, Shimoda T (2003) Clinicopathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer 6(1):39–48
Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G (2002) Management of malignant gastrointestinal stromal tumours. Lancet Oncol 3(11):655–664
Reece-Smith AM, MacGoey P, Shah MA, Leeder P, Andrew DR, McCulloch T, Parsons SL (2012) A multi-centre analysis of the impact of updated risk stratification on follow-up of gastric gastro-intestinal stromal tumours in the post-imatinib era. Eur J Surg Oncol 38(6):484–489
Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, von Mehren M (2009) Phase II trial of neoadjuvant/ adjuvant imatinib mesylate (IM) for advanced primary and metastatic/ recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 99(1):42–47
Blesius A, Cassier PA, Bertucci F, Fayette J, Ray-Coquard I, Bui B, Adenis A, Rios M, Cupissol D, Perol D, Blay JY, Le Cesne A (2011) Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer 11:72
Kodera Y, Fujiwara M, Ohashi N, Nakayama G, Koike M, Morita S, Nakao A (2010) Laparoscopic surgery for gastric cancer: a collective review with meta-analysis of randomized trials. J Am Coll Surg 211(5):677–686
Feng B, Sun J, Ling TL, Lu AG, Wang ML, Chen XY, Ma JJ, Li JW, Zang L, Han DP, Zheng MH (2012) Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: feasibility and technical strategies. Surg Endosc 26(12):3669–3675
Bagshaw PF, Allardyce RA, Frampton CM, Frizelle FA, Hewett PJ, McMurrick PJ, Rieger NA, Smith JS, Solomon MJ, Stevenson AR (2012) Long-term outcomes of the Australasian Randomized Clinical Trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study Trial. Ann Surg 256(6):915–919
Acknowledgments
We thank the follow-up office established by the Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou, Fujian Province, China. Supported by National Key Clinical Specialty Discipline Construction Program of China (No. [2012] 649).
Disclosures
All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, J., Huang, C., Zheng, C. et al. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): a size-matched comparison. Surg Endosc 28, 2577–2583 (2014). https://doi.org/10.1007/s00464-014-3506-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3506-x